3D Printing Medical Devices Market Size by Component (3D Printer, 3D Bioprinter, Material, Software, Service), Technology (EBM, DMLS, SLS, SLA, DLP, Polyjet), Application (Surgical Guides, Prosthetics, Implants), End User & Region - Global Forecast to 2028
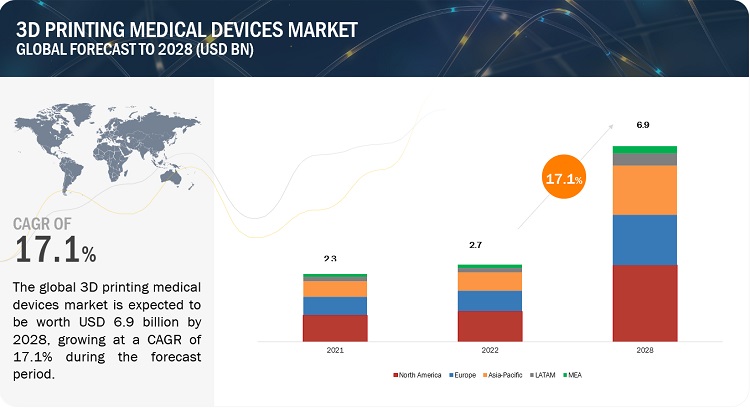
The global size of 3D printing medical devices market in terms of revenue was estimated to be worth USD 2.7 billion in 2022 and is poised to reach USD 6.9 billion by 2028, growing at a CAGR of 17.1% from 2022 to 2028. The research study consists of industry trends, pricing analysis, patent analysis, conference and webinar materials, key stakeholders, and buying behaviour in the market.
Continuous technological advancements in 3D printing have significantly improved the quality, speed, and efficiency of the devices. Innovations in materials, printing processes, and software have expanded the range of applications and made 3D printing more accessible to various industries.
To know about the assumptions considered for the study, Request for Free Sample Report
3D Printing Medical Devices Market Dynamics
Driver: Easy development of customized medical products using 3D printing
3D printing facilitates the development of customized medical products at a low cost. It directly creates 3D products based on specific CAD images of the systems in a layer-wise manner and eases the building of complex custom products with unimaginable accuracy in a small production run as compared to traditional manufacturing approaches.
In the medical field, 3D printing leads to the development of patient-specific products such as implants, hearing aids, bone scaffolds, and surgical equipment directly from patient CAD images. These custom products result in a faster recovery of patients, increased success rate of surgeries, and lower costs. Standard implants do not often suit patient requirements, especially in neurosurgery, where every human skull has an irregular and different shape, making it difficult for cranial implants. A 3D-printed patient-specific model enables physicians to plan elective surgical procedures, especially those which are complex to perform. These models optimize procedural time and improve the quality of the patient’s treatment and care. Hence, 3D printing technology is being widely used to make complex and customized prosthetic limbs, surgical implants, and brain designs.
3D Systems Corporation (US), Materialise NV (Belgium), and EOS GmbH (Germany) are some of the key players involved in providing technologies for creating patient-specific customized products. For instance, in March 2017, Johnson & Johnson (US) developed 3D-printed patient-specific surgical tools for surgeons across the US. This will enable doctors to 3D print patient-specific tools during surgical procedures, with the aim to reduce the use of multiple instruments of different sizes in operating rooms. The company’s 3D Printing Center of Excellence has partnered with DePuy Synthes (US) and Ethicon (US) for the development of prototypes for bioprinted knee meniscus tissue implantation as well as titanium alloys for cancer patients suffering from bone degradation.
The need for biocompatible and drug contact materials has increased the production of 3D-printed medical devices perfectly suited for particular individuals. Customized 3D printing allows the simultaneous production of devices along with improved manufacturing efficiency. Dental laboratories and hearing aid manufacturers are involved in the mass production of customized products. For instance, the hearing aid industry is known for the highest installations of customized consumer devices produced by 3D printing. EnvisionTEC’s E-Shell is a biocompatible material that offers over 15 shades of colors and has perspiration- and water-resistant capabilities, which is used for making custom patient solutions for hearing aid devices.
The growing demand for personalized medical products and increasing efforts by market players to develop 3D printing solutions for customized medical devices are positively impacting the growth of the global healthcare 3D printing industry.
Restraint: Stringent regulatory process for the approval of 3D-printed medical devices
Stringent regulatory guidelines (especially in the US) are a major factor restraining the growth of the 3D printing medical devices market. In the US, it takes around 3 to 7 years to prove the medical safety of any new device. As per law, even a new size of a previously approved device needs to go through the entire process before commercialization. If a customized product/device is manufactured, the company has to go through a long process in which every single part of the device has to be tested and approved individually. Simple 3D-printed devices receive FDA approval, but complex devices that need to comply with a large number of FDA requirements are a hurdle for the availability of 3D-printed products on a large scale. Moreover, state legal requirements and manufacturing regulations become an obstacle for dispensing 3D-printed medicines.
In 2016, the US FDA issued draft guidance on the Technical Considerations for Additive Manufactured Devices, with the aim to advise manufacturers engaged in developing devices using the 3D printing technique. The draft is an initial publication to obtain public feedback, and the FDA is still working on its finalization process. The FDA is also evaluating the submissions for 3D-printed medical devices with the aim to determine their safety and effectiveness. As per these requirements, the safety and effectiveness of any medical product would be determined through traditional FDA submission and preview procedures. However, to establish product safety, the device produced using the additive manufacturing technology may need additional or different testing standards compared to products manufactured through traditional (or subtractive) techniques. Furthermore, the FDA intends to provide clarity on the requirements of design controls and systems for the complaint handling of 3D printing manufacturing settings. It also aims to provide clarification on several other quality control issues, such as material qualifications and sterility.
The FDA usually evaluates all medical devices for safety and effectiveness and conducts appropriate benefit and risk assessments. However, in some cases, the FDA may require manufacturers to provide additional data, depending on the complexity of the device. At present, the FDA regulates 3D-printed medical devices in the same manner as traditionally manufactured medical products. However, 3D printing requires software and material specifications, which are stricter as compared to the typical specifications of a traditionally produced device.
Opportunity: Increasing adoption of CAD/CAM technology and desktop printers
3D printing in healthcare has evolved as per the requirements and size of healthcare facilities. Industrial-grade high-throughput 3D printers offer good capacity and quality for large healthcare facilities that need to produce large volumes of appliances on a daily basis. Desktop printers, on the other hand, offer great print quality and are smaller and versatile, with lower throughput. Affordability and the increasing adaptability and versatility of 3D printers for different types of practices have resulted in their rising adoption in dental clinics and hospitals. This has boosted the overall market growth for 3D printers for the medical and dental sector.
Computer-aided design (CAD)/computer-aided manufacturing (CAM) is increasingly being embraced by the medical and dental industry due to its high precision in dental restoration and surgical planning. This technology is used for not only designing and manufacturing milled crowns and bridges & anatomical models but also designing fabricated abutments used in dental & surgical procedures. CAD/CAM is highly useful in customizing dental (dental crowns made of zirconium) and orthopedic prosthetics. The use of CAD/CAM also reduces the need to wear temporary bridges/crowns or revision surgeries during the course of the treatment and the number of doctor visits, thereby reducing the cost of dental restoration or medical treatment procedures.
Customized CAD-based products offer superior surgical outcomes along with a significant reduction in operation time, lower risk of post-surgical complications, and improved mechanical protection to surrounding tissues or organs. According to the American Academy of Cosmetic Dentistry, in 2022, 30% of surveyed practices in the US reported using chairside CAD/CAM, up from 28% in 2019. Among practices without a chairside CAD/CAM, 32% are considering a future purchase.
Challenge: Socio-ethical concerns related to the use of 3D-printed products
3D printing is used for the development of products for a wide range of medical applications. It is used on a large scale in developing tissues and organs, with the aim to cater to the growing demand for organ transplantation across the globe. Living cells and biomaterials are used in the 3D printing process for the development of human organs. However, the use of 3D-printed products (developed using living cells) inside the human body leads to biosafety concerns. This aspect also raises ethical issues, as several religions across the world are against the use of living cells, such as stem cells, in medicine.
To monitor the activities related to the use of human embryos, strict regulations have been formulated by authorities, such as the Human Tissue Authority (HTA), Human Fertilization and Embryology Authority (HFEA), Medicines and Healthcare Products Regulatory Agency (MHRA), and the Central Ethics Committee for Stem Cell Research. The source of biomaterials used for developing 3D-printed products and concerns related to waste elimination are two other major factors hindering the adoption of 3D printing in the medical device industry.
By component, the services & software segment accounted for the largest share of the 3d printing medical devices market during the forecast period.
This segment is projected to reach USD 3.2 billion by 2028 from USD 1.4 billion in 2023, at a CAGR of 17.0% during the forecast period. Based on component, the 3D printing medical devices market is segmented into equipment, materials, and software & services. The software & services segment accounted for the largest share of the 3D printing medical devices market. However, the materials segment is estimated to grow at the highest CAGR of 17.1% during the forecast period.
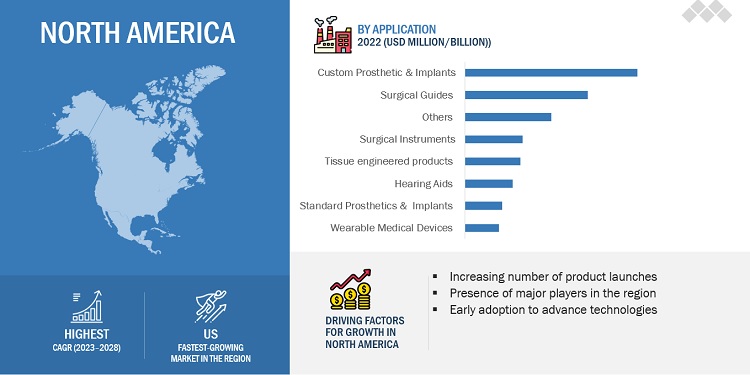
By Application, Custom Prosthetic & Implants segment holds the largest share of the global 3d printing medical devices market.
This segment is projected to reach USD 1.9 billion by 2028 from USD 0.88 billion in 2023, at a CAGR of 17.1% during the forecast period. Custom or patient-specific implants have several benefits over standard implants—they make surgeries easier, save time and cost, and reduce the margin for errors. According to industry experts, the opportunities presented by this sector—the 3D printing of custom implants—are attracting many small companies and new investors to this market.
By Technology, the laser beam melting segment accounted for the largest share of the global 3d printing medical devices market during the forecast period.
This segment is projected to reach USD 2.0 billion by 2028 from USD 0.8 bllion in 2023, at a CAGR of 18.0% during the forecast period. On the basis of technology, the 3D printing medical devices market is divided into six segments, namely, electron beam melting (EBM), laser beam melting (LBM), photopolymerization, droplet deposition or extrusion-based technologies, three-dimensional printing (3DP) or adhesion bonding, and other technologies. The laser beam melting segment is estimated to grow at the highest CAGR during the forecast period owing to the increasing application of this technology in the dental industry and for manufacturing parts for minimally invasive surgery.
By End User, the Hospitals & Surgical centers segment of the global 3d printing medical devices market is expected to grow at the fastest rate during the forecast period.
This segment is projected to reach USD 2.3 billion by 2028 from USD 1.0 billion in 2023, at a CAGR of 17.2% during the forecast period.The large share of this end-user segment can be attributed to the increasing number of 3D printing laboratories, the expansion of existing 3D printing laboratories, the outsourcing of certain manufacturing functions to 3D printing vendors, and the increasing demand for customized/fabricated solutions.
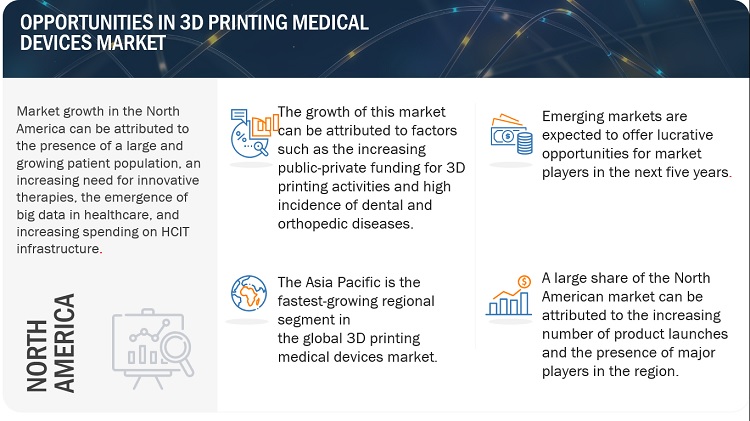
North America region of the 3d printing medical devices market is expected to be the largest market during the forecast period.
North America accounted for the largest share of 45.0% of the global 3D Printing medical devices market. The large share of the North American market is attributed to the high adoption of advanced diagnostic technologies and technological advancements. Europe accounted for the second-largest share of 29.5% of the global 3D Printing medical devices market in 2021.The Asia Pacific market is estimated to grow at the highest CAGR of 7.5% during the forecast period.
To know about the assumptions considered for the study, download the pdf brochure
As of 2022, prominent players in the market include are Stratasys Ltd. (Israel), EnvisionTEC (US), 3D Systems, Inc. (US), EOS (US), Renishaw plc (UK),GE additive(US),Desktop Metal,Inc(US), CELLINK(Sweden), Formalabs(US), , Materialise (Belgium), 3T Additive Manufacturing Ltd. (US), GENERAL ELECTRIC COMPANY (US), Carbon, Inc. (US), Prodways Group (France), SLM Solutions (Germany), Organovo Holdings Inc. (US), FIT AG (Germany), Wacker Chemie AG (Germany), Denstply Sirona(USA), DWS Systems SRL(Italy), Roland DG(Japan), HP,Inc(USA), regenHU(Switzerland), Fluicell(Sweden), Proto Labs(US), GESIM(Germany), Triastek(China), Inventia(Australia), FabRx(UK) and Apprecia Pharmaceuticals(US).
3D Printing Medical Devices Market Report Scope
|
Report Metric |
Details |
|
Market Revenue in 2022 |
$2.7 billion |
|
Projected Revenue by 2028 |
$6.9 billion |
|
Revenue Rate |
Poised to grow at a CAGR of 17.1% |
|
Market Driver |
Easy development of customized medical products using 3D printing |
|
Market Opportunity |
Increasing adoption of CAD/CAM technology and desktop printers |
This report categorizes the global 3D Printing Medical Devices Market to forecast revenue and analyze trends in each of the following submarkets:
By Component
-
Equipment
- 3D Printers
- 3D Bioprinters
-
Materials
-
Plastics
- Thermoplastics
- Photopolymers
- Metals and Metal Alloys
- Biomaterials
- Ceramics
- Paper
- Wax
- Other Materials
-
Plastics
- Services & Software
By Application
-
Surgical Guides
- Dental Guides
- Craniomaxillofacial Guides
- Orthopedic Guides
- Spinal Guides
-
Surgical Instruments
- Surgical Fasteners
- Scalpels
- Retractors
- Standard Prosthetics & Implants
-
Custom Prosthetics & Implants
- Orthopedic Implants
- Dental Prosthetics & Implants
- Craniomaxillofacial Implants
-
Tissue-engineered Products
- Bone & Cartilage Scaffolds
- Ligament & Tendon Scaffolds
- Hearing Aids
- Wearable Medical Devices
- Other Applications
By Technology
-
Laser Beam Melting
- Direct Metal Laser Sintering
- Selective Laser Sintering
- Selective Laser Melting
- LaserCUSING
-
Photopolymerization
- Digital Light Processing
- Stereolithography
- Two-photon Polymerization
- PolyJet 3D Printing
-
Droplet Deposition/Extrusion-based Technologies
- Fused Deposition Modeling
- Multiphase Jet Solidification
- Low-temperature Deposition Manufacturing
- Microextrusion Bioprinting
- Electron Beam Melting
- Three-dimensional Printing/Adhesion Bonding/Binder Jetting
- Other Technologies
By End User
- Hospitals & Surgical Centers
- Dental & Orthopedic Clinics
- Academic Institutions & Research Laboratories
- Pharma-Biotech & Medical Device Companies
- Clinical Research Organizations
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe (RoE)
-
Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- RoAPAC
-
Latin America
- Brazil
- Mexico
- RoLA
- Middle East & Africa
Recent Developments
- In Feb 2023 Stratasys Ltd launched TrueDent resin, which is used in labs for the application in dental structure shades.
- In May 2023 CELLINK launched Lumen X a new benchtop DLP bioprinter.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the 3D printing medical devices market?
The 3D printing medical devices market boasts a total revenue value of $6.9 billion by 2028.
What is the estimated growth rate (CAGR) of the 3D printing medical devices market?
The global 3D printing medical devices market has an estimated compound annual growth rate (CAGR) of 17.1% and a revenue size in the region of $2.7 billion in 2022.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the 3D printing medical devices market. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in 3D printing industry. The primary sources from the demand side include medical OEMs, Oncologists, CDMOs and service providers, among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
A breakdown of the primary respondents is provided below:
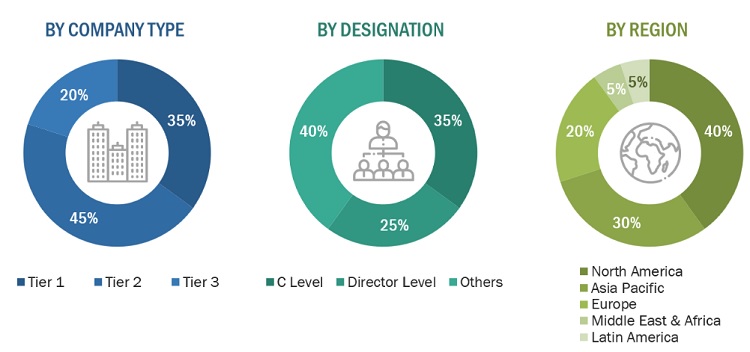
*Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
Note: Companies are classified into tiers based on their total revenue. As of 2021, Tier 1 = >USD 1,000 million, Tier 2 = USD 500–1,000 million, and Tier 3 = <USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Estimation Methodology
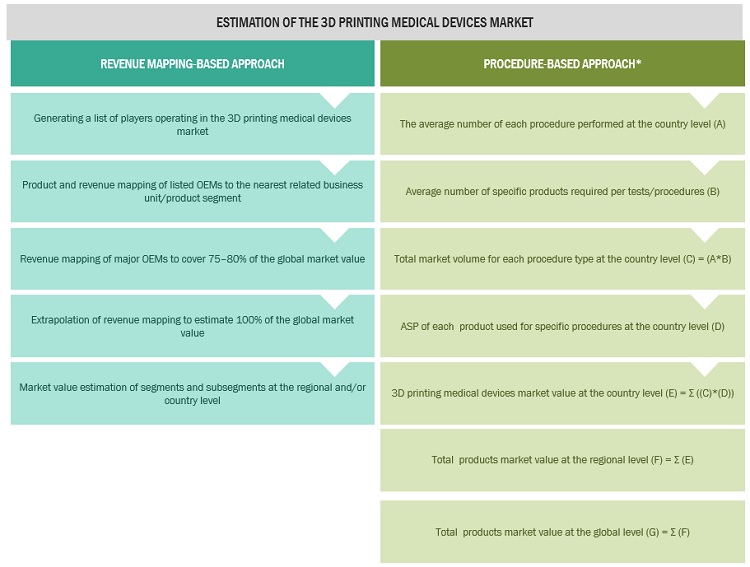
In this report, the 3D printing medical devices market size was arrived at by using the revenue share analysis of leading players. For this purpose, key players in the market were identified, and their revenues from the 3D printing segment were determined through various insights gathered during the primary and secondary research phases. Secondary research included the study of the annual and financial reports of the top market players. In contrast, primary research included extensive interviews with key opinion leaders, such as CEOs, directors, and key marketing executives.
To calculate the global market value, segmental revenues were calculated based on the revenue mapping of major solution/service providers. This process involved the following steps:
- Generating a list of major global players operating in the 3D printing devices market.
- Mapping annual revenues generated by major global players from the product segment (or nearest reported business unit/product category)
- Revenue mapping of major players to cover a major share of the global market share, as of 2021.
- Extrapolating the global value of the 3D printing medical devices market.
Approaches: Bottom-up Approach
To know about the assumptions considered for the study, Request for Free Sample Report
- For estimating the size of the 3D Printing medical devices market, the country-level market revenues were obtained from secondary sources and through extensive primary interviews.
- Country-level markets for the US and Canada were added to arrive at the market size for North America. Similarly, country-level markets for other countries were added to arrive at the market size of Europe and Asia Pacific.
- The total market derived through the bottom-up approach was again validated through secondary sources and primary sources.
Top-down approach

- The overall market size derived from the bottom-up approach was used in the top-down procedure to estimate the size of the subsegment.
- Percentage splits were applied to the total market size (splits were obtained from secondary and primary research) to obtain the market size for each subsegment.
- The top-down approach was used to reach the regional and country-level market size for these subsegments.
Data Triangulation
After arriving at the overall market size from the market size estimation process explained above, the 3D printing medical devices was split into segments and subsegments. Data triangulation and market breakdown procedures were employed to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the 3D printing medical devices market was validated using both top-down and bottom-up approaches.
Market definition
3D printing or additive manufacturing is the process of manufacturing three-dimensional solid objects from a digital file. In this technique, the objects are produced by laying down materials in the form of thin-sliced layers. A number of materials, such as plastics, metal & metal alloy powders, bioprinting biomaterials, ceramics, nylon, and paper, are utilized to develop 3D-printed products.
3D printing is adopted for many applications, including the production of physical models for medical devices for preclinical testing and prototyping, anatomy models for clinical training and clinical testing, orthodontics, prosthodontics, surgery, tissue engineering, manufacturing of dental and medical implants, construction of surgical guides and instruments, and the fabrication/customization of frameworks for prosthetics and hearing aids.
Key stakeholders
- 3D printing medical devices manufacturing companies.
- Healthcare service providers (Including hospitals, transplant centers and blood transfusion centers)
- Blood, tissue, and stem cell banks
- Government Organizations
- Independent association and regulatory authorities
- R&D companies
- Independent reference laboratories
- Diagnostic Laboratories
- Clinical Research organizations
Objectives of the Study
- To define, describe, and forecast the 3D printing medical devices market based on Component, Software &Services, Application, End User & Region.
- To provide detailed information regarding the major factors influencing the growth potential of the 3D printing medical devices market (drivers, restraints, opportunities, challenges, and trends)
- To analyze the micro markets with respect to individual growth trends, prospects, and contributions to the 3D printing medical devices market.
- To analyze key growth opportunities in the 3D printing medical devices market for key stakeholders and provide details of the competitive landscape for market leaders.
- To forecast the size of market segments and/or subsegments with respect to five major regions, namely, North America (US and Canada), Europe (Germany, France, the UK, Italy, Spain, and the RoE), Asia Pacific (Japan, China, India, Thailand, Indonesia and the RoAPAC), Latin America (Brazil, Mexico, and RoLATAM), and the Middle East & Africa
- To profile the key players in the 3D printing medical devices market and comprehensively analyze their market shares and core competencies.
- To track and analyze the competitive developments undertaken in the 3D printing medical devices market , such as product launches, agreements, expansions, and mergers & acquisitions.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the present 3D printing medical devices market
Product Analysis
- Product matrix, which gives a detailed comparison of the product portfolios of the top five companies.
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Geographic Analysis
- Further breakdown of the Rest of Europe 3D printing medical devices marketinto Russia, Belgium, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal among other
- Further breakdown of the Rest of the Asia Pacific 3D printing medical devices marketinto Singapore, Taiwan, New Zealand, Philippines, Malaysia, and other APAC countries
- Further breakdown of the Latin American 3D printing medical devices marketinto Colombia, Chile, Argentina, and Peru, among other



 Generating Response ...
Generating Response ...







Growth opportunities and latent adjacency in 3D Printing Medical Devices Market