TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 45)
1.1 STUDY OBJECTIVES
1.2 ECLINICAL SOLUTIONS INDUSTRY DEFINITION
1.2.1 INCLUSIONS AND EXCLUSIONS
1.2.2 MARKET SEGMENTATION
1.2.3 GEOGRAPHIES COVERED
1.2.4 YEARS CONSIDERED
1.3 CURRENCY CONSIDERED
1.4 LIMITATIONS
1.5 MAJOR MARKET STAKEHOLDERS
1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 51)
2.1 RESEARCH APPROACH
2.2 RESEARCH METHODOLOGY STEPS
FIGURE 1 ECLINICAL SOLUTIONS MARKET: RESEARCH METHODOLOGY
2.3 RESEARCH METHODOLOGY DESIGN
FIGURE 2 GLOBAL MARKET: RESEARCH DESIGN
2.3.1 SECONDARY DATA
2.3.1.1 Secondary sources
2.3.2 PRIMARY DATA
FIGURE 3 PRIMARY SOURCES
2.3.2.1 Insights from primary experts
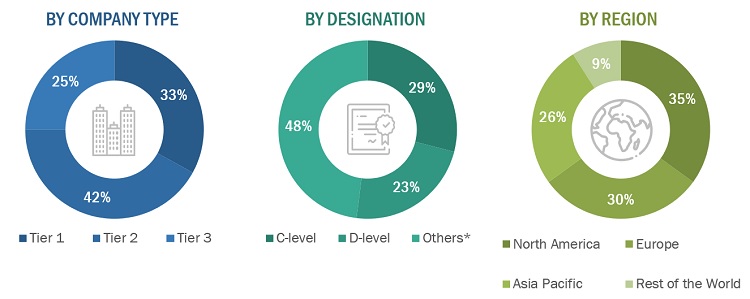
FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY, DESIGNATION, AND REGION
2.4 MARKET SIZE ESTIMATION
FIGURE 5 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
FIGURE 6 SUPPLY-SIDE MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS
FIGURE 7 REVENUE SHARE ANALYSIS ILLUSTRATION
FIGURE 8 REVENUE ANALYSIS OF TOP 3 PUBLIC COMPANIES IN ECLINICAL SOLUTIONS MARKET (2021)
FIGURE 9 BOTTOM-UP APPROACH
FIGURE 10 GLOBAL MARKET: CAGR PROJECTION FROM ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES (2022–2027)
FIGURE 11 CAGR PROJECTION: SUPPLY-SIDE ANALYSIS
FIGURE 12 TOP-DOWN APPROACH
2.5 MARKET BREAKDOWN & DATA TRIANGULATION
FIGURE 13 DATA TRIANGULATION METHODOLOGY
2.6 STUDY ASSUMPTIONS
2.7 LIMITATIONS
2.7.1 METHODOLOGY-RELATED LIMITATIONS
2.7.2 SCOPE-RELATED LIMITATIONS
2.8 RISK ASSESSMENT
TABLE 1 GLOBAL MARKET: RISK ASSESSMENT
2.9 RECESSION IMPACT ON GLOBAL MARKET
TABLE 2 GLOBAL INFLATION RATE PROJECTION, 2021–2027 (% GROWTH)
TABLE 3 US HEALTH EXPENDITURE, 2019–2022 (USD MILLION)
TABLE 4 US HEALTH EXPENDITURE, 2023–2027 (USD MILLION)
3 EXECUTIVE SUMMARY (Page No. - 67)
FIGURE 14 ECLINICAL SOLUTIONS MARKET SIZE, BY PRODUCT, 2022 VS. 2027 (USD MILLION)
FIGURE 15 GLOBAL MARKET, BY DELIVERY MODE, 2022 VS. 2027 (USD MILLION)
FIGURE 16 GLOBAL MARKET, BY CLINICAL TRIAL PHASE, 2022 VS. 2027 (USD MILLION)
FIGURE 17 GLOBAL MARKET, BY END USER, 2022 VS. 2027 (USD MILLION)
FIGURE 18 GLOBAL ECLINICAL SOLUTIONS INDUSTRY: REGIONS COVERED (USD MILLION)
4 PREMIUM INSIGHTS (Page No. - 71)
4.1 ECLINICAL SOLUTIONS MARKET OVERVIEW
FIGURE 19 INCREASING R&D EXPENDITURE ON DRUG DISCOVERY & DEVELOPMENT BY PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES TO DRIVE MARKET
4.2 NORTH AMERICA: ECLINICAL SOLUTIONS INDUSTRY, BY DELIVERY MODE & COUNTRY (2021)
FIGURE 20 WEB-HOSTED DELIVERY MODEL ACCOUNTED FOR LARGEST SHARE IN 2021
4.3 GEOGRAPHIC GROWTH OPPORTUNITIES
FIGURE 21 CHINA IS EXPECTED TO ACCOUNT FOR HIGHEST GROWTH RATE DURING FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 73)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 22 ECLINICAL SOLUTIONS MARKET SIZE: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
5.2.1 DRIVERS
5.2.1.1 Rising operational costs and regulatory requirements associated with clinical research studies
5.2.1.2 Growing adoption of novel software solutions in clinical research
5.2.1.3 Favorable government support and funding for clinical trials
5.2.1.4 Rising need for improved data standardization
5.2.1.5 Increasing R&D expenditure on drug development by pharma-biotech companies
5.2.2 RESTRAINTS
5.2.2.1 High implementation costs associated with eClinical solutions
5.2.2.2 Shortage of skilled professionals
5.2.2.3 Limited awareness among researchers
5.2.3 OPPORTUNITIES
5.2.3.1 Increasing clinical trials in emerging countries
5.2.3.2 Growing outsourcing of clinical trial processes to CROs
5.2.3.3 Gradual shift from manual data interpretation to real-time data analysis
5.2.4 CHALLENGES
5.2.4.1 Limited adoption in emerging countries due to budgetary constraints
5.2.4.2 Concerns regarding software reliability
5.2.4.3 Issues associated with patient privacy
5.3 GLOBAL MARKET: ECOSYSTEM ANALYSIS
FIGURE 23 ECOSYSTEM ANALYSIS
5.4 CASE STUDIES
5.4.1 YORK TEACHING HOSPITAL EXPEDITES DATA CAPTURE PROCESSING DURING PHASE III CLINICAL STUDY
5.4.2 LSHTM AND MENAFRICAR INCORPORATE DATA CAPTURE SOFTWARE TO STUDY MENINGOCOCCAL CARRIAGE IN AFRICA
5.4.3 CLINICAL TRIALS GROUP AT UCL UTILIZES EDC SYSTEM TO CAPTURE AND VERIFY PATIENT FEEDBACK
5.5 VALUE CHAIN ANALYSIS
FIGURE 24 GLOBAL MARKET: VALUE CHAIN ANALYSIS
5.6 PORTER'S FIVE FORCES ANALYSIS
TABLE 5 HIGH CAPITAL INVESTMENTS TO RESTRICT ENTRY OF NEW PLAYERS
5.6.1 THREAT OF NEW ENTRANTS
5.6.2 THREAT OF SUBSTITUTES
5.6.3 BARGAINING POWER OF BUYERS
5.6.4 BARGAINING POWER OF SUPPLIERS
5.6.5 INTENSITY OF COMPETITIVE RIVALRY
5.7 REGULATORY LANDSCAPE
5.7.1 NORTH AMERICA
5.7.2 EUROPE
5.7.3 ASIA PACIFIC
5.7.4 MIDDLE EAST & AFRICA
5.7.5 LATIN AMERICA
5.8 TECHNOLOGY ANALYSIS
TABLE 6 TECHNOLOGICAL DEVELOPMENTS BY LEADING VENDORS
5.9 INDUSTRY TRENDS
5.9.1 RISING NUMBER OF DECENTRALIZED CLINICAL TRAILS
5.9.2 GROWING ADOPTION OF TELEMEDICINE
5.9.3 IMPACT OF BIG DATA ON HEALTHCARE
5.10 PRICING ANALYSIS
5.10.1 AVERAGE SELLING PRICE TREND ANALYSIS
TABLE 7 AVERAGE SELLING PRICE OF ECLINICAL SOLUTIONS, BY LICENSE
5.11 KEY CONFERENCES AND EVENTS FROM 2022−2023
TABLE 8 GLOBAL MARKET: LIST OF CONFERENCES AND EVENTS FROM 2022−2023
5.12 KEY STAKEHOLDERS AND BUYING CRITERIA
FIGURE 25 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS AMONG END USERS
TABLE 9 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS AMONG END USERS
5.12.1 BUYING CRITERIA
FIGURE 26 KEY BUYING CRITERIA FOR END USERS
TABLE 10 KEY BUYING CRITERIA FOR END USERS
6 ECLINICAL SOLUTIONS MARKET SIZE, BY PRODUCT (Page No. - 91)
6.1 INTRODUCTION
TABLE 11 GLOBAL ECLINICAL SOLUTIONS INDUSTRY, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 12 GLOBAL MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
6.2 ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS
6.2.1 UTILIZATION OF COST-EFFECTIVE ECLINICAL SOLUTIONS INTEGRATED WITH EDC & CDM SYSTEMS TO DRIVE MARKET
TABLE 13 GLOBAL MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 14 GLOBAL MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 15 NORTH AMERICA: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 16 NORTH AMERICA: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 17 EUROPE: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 18 EUROPE: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 19 ASIA PACIFIC: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 20 ASIA PACIFIC: MARKET FOR ELECTRONIC DATA CAPTURE & CLINICAL DATA MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.3 CLINICAL TRIAL MANAGEMENT SYSTEMS
6.3.1 ABILITY TO BE DEPLOYED AS A SECURE WEB APPLICATION TO DRIVE UPTAKE
TABLE 21 GLOBAL MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 22 GLOBAL MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 23 NORTH AMERICA: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 24 NORTH AMERICA: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 25 EUROPE: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 26 EUROPE: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 27 ASIA PACIFIC: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 28 ASIA PACIFIC: MARKET FOR CLINICAL TRIAL MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.4 CLINICAL ANALYTICS PLATFORMS
6.4.1 ABILITY TO AUTOMATE CLINICAL DEVELOPMENT ACTIVITIES TO DRIVE MARKET
TABLE 29 GLOBAL MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 30 GLOBAL MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 31 NORTH AMERICA: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 32 NORTH AMERICA: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 33 EUROPE: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 34 EUROPE: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 35 ASIA PACIFIC: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 36 ASIA PACIFIC: MARKET FOR CLINICAL ANALYTICS PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.5 RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS
6.5.1 EFFECTIVE MANAGEMENT OF INVENTORY MONITORING AND RANDOMIZED PATIENT POOLS TO DRIVE ADOPTION
TABLE 37 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 38 GLOBAL MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 39 NORTH AMERICA: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 40 NORTH AMERICA: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 41 EUROPE: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 42 EUROPE: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 43 ASIA PACIFIC: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 44 ASIA PACIFIC: MARKET FOR RANDOMIZATION & TRIAL SUPPLY MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.6 CLINICAL DATA INTEGRATION PLATFORMS
6.6.1 ABILITY TO OPTIMIZE SECURE EXCHANGE OF EHR ON A USER-FRIENDLY GUI TO FUEL MARKET
TABLE 45 GLOBAL MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 46 GLOBAL MARKET FOR CLINICAL TRIAL INTEGRATION PLATFORMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 47 NORTH AMERICA: GLOBAL MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 48 NORTH AMERICA: GLOBAL MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 49 EUROPE: MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 50 EUROPE: MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 51 ASIA PACIFIC: MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 52 ASIA PACIFIC: MARKET FOR CLINICAL DATA INTEGRATION PLATFORMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.7 ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS
6.7.1 INCREASING ADOPTION OF EPRO SOLUTIONS AND EDIARIES AMONG PHARMA COMPANIES TO DRIVE UPTAKE
TABLE 53 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY REGION, 2016–2019 (USD MILLION)
TABLE 54 GLOBAL MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 55 NORTH AMERICA: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 56 NORTH AMERICA: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 57 EUROPE: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 58 EUROPE: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 59 ASIA PACIFIC: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 60 ASIA PACIFIC: MARKET FOR ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
6.8 SAFETY SOLUTIONS
6.8.1 ABILITY TO STREAMLINE SAFETY PROCESSING PROCESSES DURING CLINICAL TRIALS TO SUPPORT MARKET GROWTH
TABLE 61 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR SAFETY SOLUTIONS, BY REGION, 2016–2019 (USD MILLION)
TABLE 62 GLOBAL MARKET FOR SAFETY SOLUTIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 63 NORTH AMERICA: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 64 NORTH AMERICA: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 65 EUROPE: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 66 EUROPE: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 67 ASIA PACIFIC: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 68 ASIA PACIFIC: MARKET FOR SAFETY SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
6.9 ELECTRONIC TRIAL MASTER FILE SYSTEMS
6.9.1 RAPID SEARCH AND RETRIEVAL OF CLINICAL TRIAL DOCUMENTS WITH ETMF TO FUEL MARKET
TABLE 69 GLOBAL MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 70 GLOBAL MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 71 NORTH AMERICA: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 72 NORTH AMERICA: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 73 EUROPE: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 74 EUROPE: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 75 ASIA PACIFIC: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 76 ASIA PACIFIC: MARKET FOR ELECTRONIC TRIAL MASTER FILE SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.10 ELECTRONIC CONSENT SOLUTIONS
6.10.1 ABILITY TO ENSURE ADHERENCE TO ICF REGULATIONS TO INCREASE ADOPTION
TABLE 77 GLOBAL MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY REGION, 2016–2019 (USD MILLION)
TABLE 78 GLOBAL MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 79 NORTH AMERICA: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 80 NORTH AMERICA: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 81 EUROPE: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 82 EUROPE: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 83 ASIA PACIFIC: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 84 ASIA PACIFIC: MARKET FOR ELECTRONIC CONSENT SOLUTIONS, BY COUNTRY, 2020–2027 (USD MILLION)
6.11 REGULATORY INFORMATION MANAGEMENT SYSTEMS
6.11.1 ABILITY TO ASSIST RESEARCHERS IN CENTRALIZING REGULATORY INFORMATION FOR OPTIMAL STANDARDIZATION TO DRIVE MARKET
TABLE 85 GLOBAL MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY REGION, 2016–2019 (USD MILLION)
TABLE 86 GLOBAL MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY REGION, 2020–2027 (USD MILLION)
TABLE 87 NORTH AMERICA: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 88 NORTH AMERICA: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 89 EUROPE: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 90 EUROPE: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 91 ASIA PACIFIC: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 92 ASIA PACIFIC: MARKET FOR REGULATORY INFORMATION MANAGEMENT SYSTEMS, BY COUNTRY, 2020–2027 (USD MILLION)
6.12 OTHER ECLINICAL SOLUTIONS
TABLE 93 OTHER ECLINICAL SOLUTIONS MARKET SIZE, BY REGION, 2016–2019 (USD MILLION)
TABLE 94 OTHER ECLINICAL SOLUTIONS MARKET SIZE, BY REGION, 2020–2027 (USD MILLION)
TABLE 95 NORTH AMERICA: MARKET, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 96 NORTH AMERICA: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 97 EUROPE: MARKET, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 98 EUROPE: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 99 ASIA PACIFIC: MARKET, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 100 ASIA PACIFIC: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
7 ECLINICAL SOLUTIONS MARKET FORECAST, BY DELIVERY MODE (Page No. - 128)
7.1 INTRODUCTION
TABLE 101 GLOBAL ECLINICAL SOLUTIONS INDUSTRY, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 102 GLOBAL MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
7.2 WEB-HOSTED MODELS
7.2.1 ENHANCED QUALITY OF CLINICAL TRIALS WITH IMPROVED PRODUCTIVITY TO DRIVE MARKET
TABLE 103 GLOBAL MARKET FOR WEB-HOSTED MODELS, BY REGION, 2016–2019 (USD MILLION)
TABLE 104 GLOBAL MARKET FOR WEB-HOSTED MODELS, BY REGION, 2020–2027 (USD MILLION)
TABLE 105 NORTH AMERICA: MARKET FOR WEB-HOSTED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 106 NORTH AMERICA: MARKET FOR WEB-HOSTEL MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 107 EUROPE: MARKET FOR WEB-HOSTED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 108 EUROPE: MARKET FOR WEB-HOSTED MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 109 ASIA PACIFIC: MARKET FOR WEB-HOSTED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 110 ASIA PACIFIC: MARKET FOR WEB-HOSTED MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
7.3 LICENSED ENTERPRISE MODELS
7.3.1 OPTIMAL CONTROL ON DEPLOYMENT AND DATA BACKUP TO SUPPORT MARKET UPTAKE
TABLE 111 GLOBAL MARKET FOR LICENSED ENTERPRISE MODELS, BY REGION, 2016–2019 (USD MILLION)
TABLE 112 GLOBAL MARKET FOR LICENSED ENTERPRISE MODELS, BY REGION, 2020–2027 (USD MILLION)
TABLE 113 NORTH AMERICA: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 114 NORTH AMERICA: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 115 EUROPE: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 116 EUROPE: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 117 ASIA PACIFIC: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 118 ASIA PACIFIC: MARKET FOR LICENSED ENTERPRISE MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
7.4 CLOUD-BASED SOLUTIONS
7.4.1 REMOTE CENTRALIZED ACCESS WITH REAL-TIME ANALYSIS TO DRIVE MARKET
TABLE 119 GLOBAL MARKET FOR CLOUD-BASED MODELS, BY REGION, 2016–2019 (USD MILLION)
TABLE 120 GLOBAL MARKET FOR CLOUD-BASED MODELS, BY REGION, 2020–2027 (USD MILLION)
TABLE 121 NORTH AMERICA: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 122 NORTH AMERICA: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 123 EUROPE: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 124 EUROPE: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 125 ASIA PACIFIC: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 126 ASIA PACIFIC: MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2020–2027 (USD MILLION)
8 ECLINICAL SOLUTIONS MARKET FORECAST, BY CLINICAL TRIAL PHASE (Page No. - 140)
8.1 INTRODUCTION
TABLE 127 ECLINICAL SOLUTIONS MARKET FORECAST, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 128 GLOBAL ECLINICAL SOLUTIONS INDUSTRY, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
8.2 PHASE I CLINICAL TRIALS
8.2.1 ADOPTION OF EDC & CDM SYSTEMS IN PHASE I STUDIES TO DRIVE MARKET
TABLE 129 GLOBAL MARKET FOR PHASE I, BY REGION, 2016–2019 (USD MILLION)
TABLE 130 ECLINICAL SOLUTIONS MARKET FORECAST FOR PHASE I, BY REGION, 2020–2027 (USD MILLION)
TABLE 131 NORTH AMERICA: MARKET FOR PHASE I, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 132 NORTH AMERICA: MARKET FOR PHASE I, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 133 EUROPE: MARKET FOR PHASE I, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 134 EUROPE: MARKET FOR PHASE I, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 135 ASIA PACIFIC: MARKET FOR PHASE I, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 136 ASIA PACIFIC: MARKET FOR PHASE I, BY COUNTRY, 2020–2027 (USD MILLION)
8.3 PHASE II CLINICAL TRIALS
8.3.1 UTILIZATION OF ECLINICAL SOLUTIONS IN STREAMLINING PHASE II TRIALS TO BOOST MARKET
TABLE 137 ECLINICAL SOLUTIONS MARKET FORECAST FOR PHASE II, BY REGION, 2016–2019 (USD MILLION)
TABLE 138 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR PHASE II, BY REGION, 2020–2027 (USD MILLION)
TABLE 139 NORTH AMERICA: MARKET FOR PHASE II, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 140 NORTH AMERICA: MARKET FOR PHASE II, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 141 EUROPE: MARKET FOR PHASE II, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 142 EUROPE: MARKET FOR PHASE II, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 143 ASIA PACIFIC: MARKET FOR PHASE II, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 144 ASIA PACIFIC: MARKET FOR PHASE II, BY COUNTRY, 2020–2027 (USD MILLION)
8.4 PHASE III CLINICAL TRIALS
8.4.1 INVOLVEMENT OF A LARGE PATIENT POPULATION TO DRIVE MARKET
TABLE 145 ECLINICAL SOLUTIONS MARKET TRENDS FOR PHASE III, BY REGION, 2016–2019 (USD MILLION)
TABLE 146 GLOBAL MARKET FOR PHASE III, BY REGION, 2020–2027 (USD MILLION)
TABLE 147 NORTH AMERICA: MARKET FOR PHASE III, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 148 NORTH AMERICA: MARKET FOR PHASE III, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 149 EUROPE: MARKET FOR PHASE III, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 150 EUROPE: MARKET FOR PHASE III, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 151 ASIA PACIFIC: MARKET FOR PHASE III, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 152 ASIA PACIFIC: MARKET FOR PHASE III, BY COUNTRY, 2020–2027 (USD MILLION)
8.5 PHASE IV CLINICAL TRIALS
8.5.1 RISING DEMAND FOR POST-MARKETING SURVEILLANCE TO SUPPORT MARKET GROWTH
TABLE 153 ECLINICAL SOLUTIONS MARKET TRENDS FOR PHASE IV, BY REGION, 2016–2019 (USD MILLION)
TABLE 154 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR PHASE IV, BY REGION, 2020–2027 (USD MILLION)
TABLE 155 NORTH AMERICA: MARKET FOR PHASE IV, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 156 NORTH AMERICA: MARKET FOR PHASE IV, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 157 EUROPE: MARKET FOR PHASE IV, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 158 EUROPE: MARKET FOR PHASE IV, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 159 ASIA PACIFIC: MARKET FOR PHASE IV, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 160 ASIA PACIFIC: MARKET FOR PHASE IV, BY COUNTRY, 2020–2027 (USD MILLION)
9 ECLINICAL SOLUTIONS MARKET TRENDS, BY END USER (Page No. - 155)
9.1 INTRODUCTION
TABLE 161 ECLINICAL SOLUTIONS MARKET TRENDS, BY END USER, 2016–2019 (USD MILLION)
TABLE 162 GLOBAL MARKET, BY END USER, 2020–2027 (USD MILLION)
9.2 PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES
9.2.1 INCREASING R&D EXPENDITURE FOR DRUG DISCOVERY AND DEVELOPMENT TO DRIVE MARKET
TABLE 163 GLOBAL ECLINICAL SOLUTIONS INDUSTRY SIZE PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY REGION, 2016–2019 (USD MILLION)
TABLE 164 GLOBAL MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY REGION, 2020–2027 (USD MILLION)
TABLE 165 NORTH AMERICA: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 166 NORTH AMERICA: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 167 EUROPE: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 168 EUROPE: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 169 ASIA PACIFIC: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 170 ASIA PACIFIC: MARKET FOR PHARMACEUTICAL & BIOPHARMACEUTICAL COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
9.3 CONTRACT RESEARCH ORGANIZATIONS
9.3.1 RISING OUTSOURCING OF CLINICAL TRIAL ACTIVITIES TO CROS BY PHARMACEUTICAL COMPANIES TO DRIVE MARKET
TABLE 171 ECLINICAL SOLUTIONS MARKET TRENDS FOR CONTRACT RESEARCH ORGANIZATIONS, BY REGION, 2016–2019 (USD MILLION)
TABLE 172 GLOBAL MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY REGION, 2020–2027 (USD MILLION)
TABLE 173 NORTH AMERICA: MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 174 NORTH AMERICA: MARKET SIZE FOR CROS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 175 EUROPE: MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 176 EUROPE: MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 177 ASIA PACIFIC: MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 178 ASIA PACIFIC: MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2020–2027 (USD MILLION)
9.4 CONSULTANCY SERVICE COMPANIES
9.4.1 GROWING COLLABORATIONS FOR CDM BETWEEN CONSULTANCY FIRMS AND PHARMA & BIOTECH COMPANIES TO DRIVE MARKET
TABLE 179 ECLINICAL SOLUTIONS MARKET GRAPH FOR CONSULTANCY SERVICE COMPANIES, BY REGION, 2016–2019 (USD MILLION)
TABLE 180 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR CONSULTANCY SERVICE COMPANIES, BY REGION, 2020–2027 (USD MILLION)
TABLE 181 NORTH AMERICA: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 182 NORTH AMERICA: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 183 EUROPE: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 184 EUROPE: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 185 ASIA PACIFIC: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 186 ASIA PACIFIC: MARKET FOR CONSULTANCY SERVICE COMPANIES, BY COUNTRY, 2020–2027 (USD MILLION)
9.5 MEDICAL DEVICE MANUFACTURERS
9.5.1 UTILIZATION OF CLINICAL DATA SOFTWARE SOLUTIONS FOR MEDICAL DEVICE TRIALS TO FUEL MARKET
TABLE 187 ECLINICAL SOLUTIONS MARKET GRAPH FOR MEDICAL DEVICE MANUFACTURERS, BY REGION, 2016–2019 (USD MILLION)
TABLE 188 GLOBAL MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY REGION, 2020–2027 (USD MILLION)
TABLE 189 NORTH AMERICA: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 190 NORTH AMERICA: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 191 EUROPE: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 192 EUROPE: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 193 ASIA PACIFIC: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 194 ASIA PACIFIC: MARKET FOR MEDICAL DEVICE MANUFACTURERS, BY COUNTRY, 2020–2027 (USD MILLION)
9.6 HOSPITALS
9.6.1 INCREASING IT BUDGETS TO STREAMLINE PATIENT DATABASES TO PROPEL MARKET
TABLE 195 ECLINICAL SOLUTIONS MARKET GRAPH FOR HOSPITALS, BY REGION, 2016–2019 (USD MILLION)
TABLE 196 GLOBAL ECLINICAL SOLUTIONS INDUSTRY FOR HOSPITALS, BY REGION, 2020–2027 (USD MILLION)
TABLE 197 NORTH AMERICA: MARKET FOR HOSPITALS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 198 NORTH AMERICA: MARKET SIZE FOR HOSPITALS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 199 EUROPE: MARKET FOR HOSPITALS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 200 EUROPE: MARKET FOR HOSPITALS, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 201 ASIA PACIFIC: MARKET FOR HOSPITALS, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 202 ASIA PACIFIC: MARKET FOR HOSPITALS, BY COUNTRY, 2020–2027 (USD MILLION)
9.7 ACADEMIC & RESEARCH INSTITUTES
9.7.1 FAVORABLE GOVERNMENT SUPPORT FOR CLINICAL RESEARCH TO SUPPORT MARKET GROWTH
TABLE 203 ECLINICAL SOLUTIONS MARKET GRAPH FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2016–2019 (USD MILLION)
TABLE 204 GLOBAL MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2020–2027 (USD MILLION)
TABLE 205 NORTH AMERICA: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 206 NORTH AMERICA: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 207 EUROPE: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 208 EUROPE: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 209 ASIA PACIFIC: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 210 ASIA PACIFIC: MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2020–2027 (USD MILLION)
10 ECLINICAL SOLUTIONS MARKET GRAPH, BY REGION (Page No. - 176)
10.1 INTRODUCTION
TABLE 211 GLOBAL ECLINICAL SOLUTIONS INDUSTRY, BY REGION, 2016–2019 (USD MILLION)
TABLE 212 GLOBAL MARKET, BY REGION, 2020–2027 (USD MILLION)
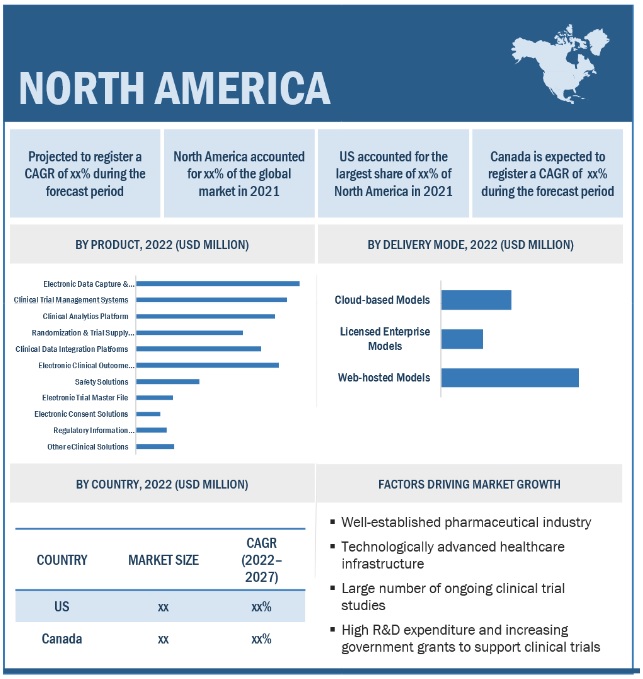
10.2 NORTH AMERICA
FIGURE 27 NORTH AMERICA: ECLINICAL SOLUTIONS MARKET SNAPSHOT
TABLE 213 NORTH AMERICA: MARKET, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 214 NORTH AMERICA: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 215 NORTH AMERICA: MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 216 NORTH AMERICA: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 217 NORTH AMERICA: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 218 NORTH AMERICA: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 219 NORTH AMERICA: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 220 NORTH AMERICA: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 221 NORTH AMERICA: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 222 NORTH AMERICA: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.2.1 NORTH AMERICA: RECESSION IMPACT
10.2.2 US
10.2.2.1 Rising government funding for pharmaceutical R&D to drive market
FIGURE 28 DISTRIBUTION OF R&D COMPANIES BY COUNTRY/REGION (2021 VS. 2022)
TABLE 223 US: ECLINICAL SOLUTIONS MARKET PREDICTION, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 224 US: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 225 US: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 226 US: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 227 US: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 228 US: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 229 US: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 230 US: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.2.3 CANADA
10.2.3.1 Availability of advanced facilities and shorter approval times for drug candidates to drive market
TABLE 231 CANADA: ECLINICAL SOLUTIONS MARKET PREDICTION, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 232 CANADA: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 233 CANADA: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 234 CANADA: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 235 CANADA: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 236 CANADA: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 237 CANADA: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 238 CANADA: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.3 EUROPE
TABLE 239 EUROPE: ECLINICAL SOLUTIONS MARKET PREDICTION, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 240 EUROPE: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 241 EUROPE: MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 242 EUROPE: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 243 EUROPE: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 244 EUROPE: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 245 EUROPE: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 246 EUROPE: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 247 EUROPE: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 248 EUROPE: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.3.1 EUROPE: RECESSION IMPACT
10.3.2 GERMANY
10.3.2.1 High number of sponsored clinical trials to drive uptake of eClinical solutions
TABLE 249 GERMANY: ECLINICAL SOLUTIONS MARKET PREDICTION, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 250 GERMANY: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 251 GERMANY: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 252 GERMANY: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 253 GERMANY: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 254 GERMANY: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 255 GERMANY: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 256 GERMANY: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.3.3 UK
10.3.3.1 Investments by pharmaceutical sponsors for drug discovery services to boost market
TABLE 257 UK: ECLINICAL SOLUTIONS MARKET PREDICTION, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 258 UK: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 259 UK: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 260 UK: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 261 UK: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 262 UK: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 263 UK: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 264 UK: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.3.4 FRANCE
10.3.4.1 Growing R&D pipeline for oncology trials to drive market
TABLE 265 FRANCE: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 266 FRANCE: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 267 FRANCE: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 268 FRANCE: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 269 FRANCE: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 270 FRANCE: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 271 FRANCE: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 272 FRANCE: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.3.5 ITALY
10.3.5.1 Increasing government funds and favorable regulatory scenarios to fuel uptake
TABLE 273 NUMBER OF CLINICAL TRIALS IN ITALY, BY COMPANY (JANUARY 2022)
TABLE 274 ITALY: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 275 ITALY: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 276 ITALY: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 277 ITALY: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 278 ITALY: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 279 ITALY: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 280 ITALY: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 281 ITALY: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.3.6 SPAIN
10.3.6.1 Established network of research centers to propel market
TABLE 282 SPAIN: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 283 SPAIN: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 284 SPAIN: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 285 SPAIN: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 286 SPAIN: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 287 SPAIN: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 288 SPAIN: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 289 SPAIN: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.3.7 REST OF EUROPE
TABLE 290 REST OF EUROPE: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 291 REST OF EUROPE: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 292 REST OF EUROPE: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 293 REST OF EUROPE: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 294 REST OF EUROPE: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 295 REST OF EUROPE: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 296 REST OF EUROPE: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 297 REST OF EUROPE: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.4 ASIA PACIFIC
FIGURE 29 ASIA PACIFIC: ECLINICAL SOLUTIONS MARKET SNAPSHOT
10.4.1 ASIA PACIFIC: RECESSION IMPACT
TABLE 298 ASIA PACIFIC: MARKET, BY COUNTRY, 2016–2019 (USD MILLION)
TABLE 299 ASIA PACIFIC: MARKET, BY COUNTRY, 2020–2027 (USD MILLION)
TABLE 300 ASIA PACIFIC: MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 301 ASIA PACIFIC: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 302 ASIA PACIFIC: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 303 ASIA PACIFIC: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 304 ASIA PACIFIC: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 305 ASIA PACIFIC: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 306 ASIA PACIFIC: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 307 ASIA PACIFIC: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.4.2 CHINA
10.4.2.1 Low cost of clinical trials and large pharmaceutical R&D base to drive market
TABLE 308 CHINA: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 309 CHINA: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 310 CHINA: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 311 CHINA: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 312 CHINA: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 313 CHINA: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 314 CHINA: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 315 CHINA: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.4.3 JAPAN
10.4.3.1 Established clinical trial infrastructure and biomedical research capabilities to support market growth
TABLE 316 JAPAN: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 317 JAPAN: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 318 JAPAN: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 319 JAPAN: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 320 JAPAN: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 321 JAPAN: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 322 JAPAN: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 323 JAPAN: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.4.4 INDIA
10.4.4.1 Growing pharmaceutical industry to fuel uptake of eClinical solutions
TABLE 324 INDIA: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 325 INDIA: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 326 INDIA: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 327 INDIA: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 328 INDIA: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 329 INDIA: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 330 INDIA: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 331 INDIA: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.4.5 REST OF ASIA PACIFIC
TABLE 332 REST OF ASIA PACIFIC: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 333 REST OF ASIA PACIFIC: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 334 REST OF ASIA PACIFIC: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 335 REST OF ASIA PACIFIC: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 336 REST OF ASIA PACIFIC: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 337 REST OF ASIA PACIFIC: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 338 REST OF ASIA PACIFIC: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 339 REST OF ASIA PACIFIC: MARKET, BY END USER, 2020–2027 (USD MILLION)
10.5 REST OF THE WORLD
TABLE 340 REST OF THE WORLD: ECLINICAL SOLUTIONS MARKET, BY PRODUCT, 2016–2019 (USD MILLION)
TABLE 341 REST OF THE WORLD: MARKET, BY PRODUCT, 2020–2027 (USD MILLION)
TABLE 342 REST OF THE WORLD: MARKET, BY DELIVERY MODE, 2016–2019 (USD MILLION)
TABLE 343 REST OF THE WORLD: MARKET, BY DELIVERY MODE, 2020–2027 (USD MILLION)
TABLE 344 REST OF THE WORLD: MARKET, BY CLINICAL TRIAL PHASE, 2016–2019 (USD MILLION)
TABLE 345 REST OF THE WORLD: MARKET, BY CLINICAL TRIAL PHASE, 2020–2027 (USD MILLION)
TABLE 346 REST OF THE WORLD: MARKET, BY END USER, 2016–2019 (USD MILLION)
TABLE 347 REST OF THE WORLD: ECLINICAL SOLUTIONS INDUSTRY, BY END USER, 2020–2027 (USD MILLION)
10.5.1 REST OF THE WORLD: RECESSION IMPACT
11 COMPETITIVE LANDSCAPE (Page No. - 252)
11.1 INTRODUCTION
11.2 STRATEGIES ADOPTED BY KEY PLAYERS
11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS
11.3 REVENUE SHARE ANALYSIS OF KEY MARKET PLAYERS
FIGURE 30 ECLINICAL SOLUTIONS MARKET: KEY PLAYERS
11.4 MARKET RANKING ANALYSIS (2021)
FIGURE 31 GLOBAL MARKET: COMPANY RANKS (2021)
11.5 COMPANY EVALUATION QUADRANT
11.5.1 STARS
11.5.2 EMERGING LEADERS
11.5.3 PERVASIVE PLAYERS
11.5.4 PARTICIPANTS
FIGURE 32 GLOBAL MARKET: COMPANY EVALUATION QUADRANT (2021)
11.6 GLOBAL ECLINICAL SOLUTIONS INDUSTRY: COMPETITIVE EVALUATION QUADRANT FOR SMES
11.6.1 PROGRESSIVE COMPANIES
11.6.2 STARTING BLOCKS
11.6.3 RESPONSIVE COMPANIES
11.6.4 DYNAMIC COMPANIES
FIGURE 33 GLOBAL MARKET: COMPANY EVALUATION QUADRANT FOR SMES (2021)
11.7 COMPETITIVE BENCHMARKING
FIGURE 34 GLOBAL MARKET: OVERALL FOOTPRINT OF 15 COMPANIES
TABLE 348 GLOBAL MARKET: COMPANY PRODUCT FOOTPRINT
TABLE 349 GLOBAL MARKET: END USER FOOTPRINT
TABLE 350 GLOBAL ECLINICAL SOLUTIONS INDUSTRY: COMPANY REGIONAL FOOTPRINT
11.8 COMPETITIVE SITUATIONS AND TRENDS
11.8.1 PRODUCT AND SERVICE LAUNCHES
TABLE 351 PRODUCT AND SERVICE LAUNCHES (2019−2022)
11.8.2 DEALS
TABLE 352 DEALS (2019−2022)
12 COMPANY PROFILES (Page No. - 265)
12.1 KEY PLAYERS
(Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))*
12.1.1 ORACLE CORPORATION
TABLE 353 ORACLE CORPORATION: BUSINESS OVERVIEW
FIGURE 35 ORACLE CORPORATION: COMPANY SNAPSHOT (2022)
12.1.2 DASSAULT SYSTÈMES
TABLE 354 DASSAULT SYSTÈMES: BUSINESS OVERVIEW
FIGURE 36 DASSAULT SYSTÈMES: COMPANY SNAPSHOT (2021)
12.1.3 PAREXEL INTERNATIONAL CORPORATION
TABLE 355 PAREXEL INTERNATIONAL CORPORATION: BUSINESS OVERVIEW
12.1.4 CLARIO
TABLE 356 CLARIO: BUSINESS OVERVIEW
12.1.5 DATATRAK INT.
TABLE 357 DATATRAK INT.: BUSINESS OVERVIEW
FIGURE 37 DATATRAK INT.: COMPANY SNAPSHOT (2021)
12.1.6 SIGNANT HEALTH (CRF HEALTH)
TABLE 358 SIGNANT HEALTH: BUSINESS OVERVIEW
12.1.7 MAXISIT INC.
TABLE 359 MAXISIT INC.: BUSINESS OVERVIEW
12.1.8 4G CLINICAL
TABLE 360 4G CLINICAL: BUSINESS OVERVIEW
12.1.9 ECLINICAL SOLUTIONS LLC.
TABLE 361 ECLINICAL SOLUTIONS: BUSINESS OVERVIEW
12.1.10 VEEVA SYSTEMS
TABLE 362 VEEVA SYSTEMS: BUSINESS OVERVIEW
FIGURE 38 VEEVA SYSTEMS: COMPANY SNAPSHOT (2021)
12.2 OTHER PLAYERS
12.2.1 RESEARCHMANAGER
12.2.2 SAAMA TECHNOLOGIES
12.2.3 MEDNET
12.2.4 ANJU SOFTWARE (PORTFOLIO COMPANY OF ABRY PARTNERS)
12.2.5 MEDRIO, INC.
12.2.6 CASTOR
12.2.7 CLINIPACE
12.2.8 IBM WATSON HEALTH
12.2.9 ARISGLOBAL
12.2.10 QURETEC LTD.
12.2.11 OPENCLINICA, LLC.
12.2.12 ADVARRA
12.2.13 REALTIME SOFTWARE SOLUTIONS, LLC
12.2.14 YPRIME LLC.
12.2.15 VIAL
*Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
13 APPENDIX (Page No. - 300)
13.1 DISCUSSION GUIDE
13.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
13.3 CUSTOMIZATION OPTIONS
13.4 RELATED REPORTS




 Generating Response ...
Generating Response ...







Growth opportunities and latent adjacency in eClinical Solutions Market
Looking to gain more insights on the global eClinical Solutions Market
What are the growth opportunities in eClinical Solutions Market?
Can you enlighten us on geographical growth analysis in eClinical Solutions Market?