TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 40)
1.1 STUDY OBJECTIVES
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS & EXCLUSIONS
1.3 MARKET SCOPE
1.3.1 MARKETS COVERED
FIGURE 1 GENE THERAPY MARKET SEGMENTATION
1.3.2 YEARS CONSIDERED
1.4 CURRENCY CONSIDERED
1.5 LIMITATIONS
1.6 STAKEHOLDERS
1.7 SUMMARY OF CHANGES
1.7.1 RECESSION IMPACT
2 RESEARCH METHODOLOGY (Page No. - 44)
2.1 RESEARCH DATA
FIGURE 2 RESEARCH DESIGN
2.1.1 SECONDARY DATA
2.1.2 PRIMARY DATA
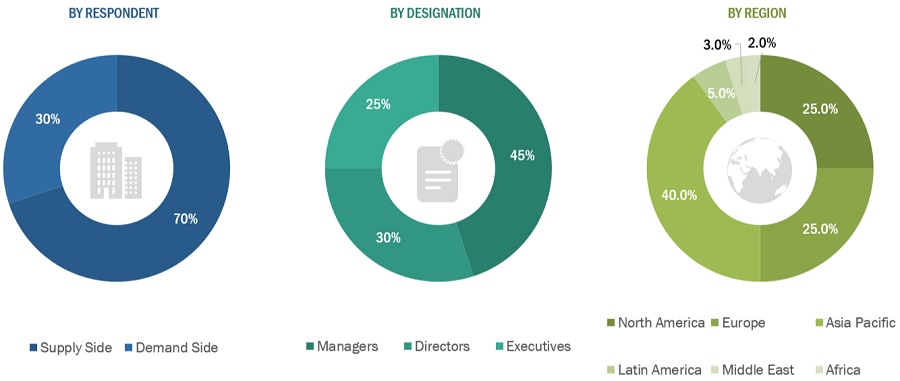
FIGURE 3 GENE THERAPY MARKET: BREAKDOWN OF PRIMARIES
2.2 MARKET SIZE ESTIMATION OF GENE THERAPY INDUSTRY
FIGURE 4 CELL AND GENE THERAPY MARKET SIZE ESTIMATION: SUPPLY-SIDE ANALYSIS
FIGURE 5 MARKET SIZE ESTIMATION FOR MARKET: REVENUE SHARE ANALYSIS
2.2.1 INSIGHTS FROM PRIMARIES
FIGURE 6 MARKET VALIDATION FROM PRIMARY EXPERTS
FIGURE 7 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
2.3 GROWTH FORECAST
FIGURE 8 MARKET: CAGR PROJECTIONS
FIGURE 9 MARKET: GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
FIGURE 10 DATA TRIANGULATION METHODOLOGY
2.5 RESEARCH ASSUMPTIONS
2.6 RISK ANALYSIS
2.7 RECESSION IMPACT ANALYSIS
TABLE 1 GLOBAL INFLATION RATE PROJECTIONS, 2021–2027 (% GROWTH)
3 EXECUTIVE SUMMARY (Page No. - 56)
FIGURE 11 GENE THERAPY MARKET, BY TYPE, 2023 VS. 2028 (USD MILLION)
FIGURE 12 GENE THERAPY INDUSTRY, BY VECTOR, 2023 VS. 2028 (USD MILLION)
FIGURE 13 CELL AND GENE THERAPY MARKET, BY THERAPEUTIC AREA, 2023 VS. 2028 (USD MILLION)
FIGURE 14 MARKET, BY DELIVERY METHOD, 2023 VS. 2028 (USD MILLION)
FIGURE 15 MARKET, BY ROUTE OF ADMINISTRATION, 2023 VS. 2028 (USD MILLION)
FIGURE 16 GEOGRAPHICAL SNAPSHOT OF MARKET
4 PREMIUM INSIGHTS (Page No. - 60)
4.1 GENE THERAPY MARKET OVERVIEW
FIGURE 17 INCREASING INVESTMENTS IN GENE THERAPY RESEARCH TO DRIVE GROWTH IN GENE THERAPY INDUSTRY
4.2 NORTH AMERICA: MARKET, BY THERAPEUTIC AREA & COUNTRY (2022)
FIGURE 18 NEUROLOGY ACCOUNTED FOR LARGEST SHARE OF NORTH AMERICAN MARKET IN 2022
4.3 CELL AND GENE THERAPY MARKET, BY VECTOR, 2022
FIGURE 19 VIRAL VECTORS SEGMENT TO DOMINATE MARKET DURING FORECAST PERIOD
4.4 MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
FIGURE 20 GERMANY TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 63)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 21 GENE THERAPY MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
5.2.1 DRIVERS
5.2.1.1 Increasing regulatory approvals for gene therapy products
TABLE 2 LIST OF KEY PRODUCT APPROVALS IN GENE THERAPY INDUSTRY, 2021–2023
5.2.1.2 Increasing investments in gene therapy research
5.2.1.3 Growing technological advancements
TABLE 3 LIST OF ADVANCED PLATFORMS LAUNCHED IN MARKET, 2022–2023
5.2.1.4 Rising prevalence of genetic disorders and cancer
5.2.2 RESTRAINTS
5.2.2.1 High cost of gene therapy products
TABLE 4 COST OF GENE THERAPY PRODUCTS
5.2.3 OPPORTUNITIES
5.2.3.1 Rising demand for cell & gene therapies
5.2.3.2 Increasing focus on precision medicine
5.2.4 CHALLENGES
5.2.4.1 Complex manufacturing process
5.2.4.2 Short shelf life and supply chain challenges
5.3 PORTER’S FIVE FORCES ANALYSIS
TABLE 5 MARKET: PORTER’S FIVE FORCES ANALYSIS
5.3.1 THREAT OF NEW ENTRANTS
5.3.2 THREAT OF SUBSTITUTES
5.3.3 BARGAINING POWER OF SUPPLIERS
5.3.4 BARGAINING POWER OF BUYERS
5.3.5 INTENSITY OF COMPETITIVE RIVALRY
5.4 TECHNOLOGY ANALYSIS
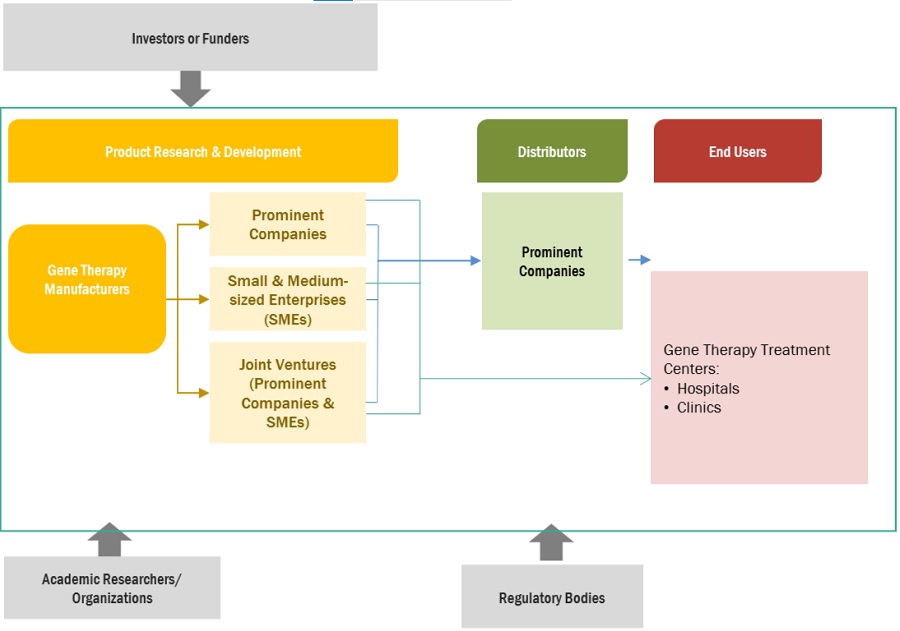
5.5 VALUE CHAIN ANALYSIS
FIGURE 22 MARKET: VALUE CHAIN
5.6 ECOSYSTEM ANALYSIS
FIGURE 23 MARKET: ECOSYSTEM MAP
5.7 PATENT ANALYSIS
FIGURE 24 PATENT APPLICATIONS FOR GENE THERAPY PRODUCTS, JANUARY 2012–SEPTEMBER 2023
TABLE 6 MARKET: INDICATIVE LIST OF PATENTS
5.8 PIPELINE ANALYSIS
TABLE 7 GENE THERAPY PRODUCTS IN CLINICAL PIPELINE
5.9 SUPPLY CHAIN ANALYSIS
FIGURE 25 HERAPY MARKET: SUPPLY CHAIN ANALYSIS
TABLE 8 SUPPLY CHAIN ECOSYSTEM
5.10 REGULATORY LANDSCAPE
5.10.1 REGULATORY ANALYSIS
5.10.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS, BY REGION
TABLE 9 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 10 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 11 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 12 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.11 PRICING ANALYSIS
5.11.1 AVERAGE SELLING PRICE TREND OF KEY PLAYERS
TABLE 13 AVERAGE SELLING PRICE OF GENE THERAPY PRODUCTS
TABLE 14 AVERAGE SELLING PRICE OF GENE THERAPY PRODUCTS, BY DELIVERY METHOD
5.12 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES
5.13 KEY CONFERENCES & EVENTS
TABLE 15 MARKET: DETAILED LIST OF CONFERENCES & EVENTS (2023–2024)
5.14 KEY STAKEHOLDERS & BUYING CRITERIA
5.14.1 KEY STAKEHOLDERS ON BUYING PROCESS
FIGURE 26 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR GENE THERAPY PRODUCTS IN HOSPITALS AND CLINICS
5.14.2 BUYING CRITERIA FOR MARKET
FIGURE 27 KEY BUYING CRITERIA FOR HOSPITALS AND CLINICS
6 GENE THERAPY MARKET, BY TYPE (Page No. - 87)
6.1 INTRODUCTION
TABLE 16 GENE THERAPY INDUSTRY, BY TYPE, 2021–2028 (USD MILLION)
6.2 GENE SILENCING
6.2.1 HIGH FLEXIBILITY AND PRECISION OFFERED BY GENE SILENCING THERAPIES TO SUPPORT GROWTH
TABLE 17 GENE SILENCING THERAPY MARKET, BY REGION, 2021–2028 (USD MILLION)
TABLE 18 NORTH AMERICA: GENE SILENCING THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 19 EUROPE: GENE SILENCING THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 20 ASIA PACIFIC: GENE SILENCING THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 21 LATIN AMERICA: GENE SILENCING THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
6.3 CELL REPLACEMENT
6.3.1 LONG-TERM EFFECTS OF CELL REPLACEMENT THERAPY TO BOOST ADOPTION
TABLE 22 CELL REPLACEMENT THERAPY MARKET, BY REGION, 2021–2028 (USD MILLION)
TABLE 23 NORTH AMERICA: CELL REPLACEMENT THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 24 EUROPE: CELL REPLACEMENT THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 25 ASIA PACIFIC: CELL REPLACEMENT THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 26 LATIN AMERICA: CELL REPLACEMENT THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
6.4 GENE AUGMENTATION
6.4.1 GROWING USE OF GENE AUGMENTATION THERAPY AGAINST MONOGENIC DISORDERS TO FAVOR MARKET GROWTH
TABLE 27 GENE AUGMENTATION THERAPY MARKET, BY REGION, 2021–2028 (USD MILLION)
TABLE 28 NORTH AMERICA: GENE AUGMENTATION THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 29 EUROPE: GENE AUGMENTATION THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 30 ASIA PACIFIC: GENE AUGMENTATION THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 31 LATIN AMERICA: GENE AUGMENTATION THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
6.5 OTHER THERAPIES
7 GENE THERAPY MARKET, BY VECTOR (Page No. - 96)
7.1 INTRODUCTION
TABLE 32 GENE THERAPY INDUSTRY, BY VECTOR, 2021–2028 (USD MILLION)
7.2 VIRAL VECTORS
TABLE 33 MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 34 MARKET FOR VIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 35 NORTH AMERICA: MARKET FOR VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 36 EUROPE: MARKET FOR VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 37 ASIA PACIFIC: MARKET FOR VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 38 LATIN AMERICA: MARKET FOR VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.2.1 RETROVIRAL VECTORS
TABLE 39 MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 40 MARKET FOR RETROVIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 41 NORTH AMERICA: MARKET FOR RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 42 EUROPE: MARKET FOR RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 43 ASIA PACIFIC: MARKET FOR RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 44 LATIN AMERICA: MARKET FOR RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.2.1.1 Gamma retroviral vectors
7.2.1.1.1 Availability of wide range of gamma-retroviral vectors to support market growth
TABLE 45 MARKET FOR GAMMA RETROVIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 46 NORTH AMERICA: MARKET FOR GAMMA RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 47 EUROPE: MARKET FOR GAMMA RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 48 ASIA PACIFIC: MARKET FOR GAMMA RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 49 LATIN AMERICA: MARKET FOR GAMMA RETROVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.2.1.2 Lentiviral vectors
7.2.1.2.1 North America to dominate lentiviral vectors market
TABLE 50 MARKET FOR LENTIVIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 51 NORTH AMERICA: MARKET FOR LENTIVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 52 EUROPE: MARKET FOR LENTIVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 53 ASIA PACIFIC: MARKET FOR LENTIVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 54 LATIN AMERICA: MARKET FOR LENTIVIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.2.2 ADENO-ASSOCIATED VIRAL VECTORS
7.2.2.1 Growing clinical pipeline of adeno-associated viral vectors to drive growth
TABLE 55 MARKET FOR ADENO-ASSOCIATED VIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 56 NORTH AMERICA: MARKET FOR ADENO-ASSOCIATED VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 57 EUROPE: MARKET FOR ADENO-ASSOCIATED VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 58 ASIA PACIFIC: MARKET FOR ADENO-ASSOCIATED VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 59 LATIN AMERICA: MARKET FOR ADENO-ASSOCIATED VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.2.3 OTHER VIRAL VECTORS
TABLE 60 MARKET FOR OTHER VIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 61 NORTH AMERICA: MARKET FOR OTHER VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 62 EUROPE: MARKET FOR OTHER VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 63 ASIA PACIFIC: MARKET FOR OTHER VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 64 LATIN AMERICA: MARKET FOR OTHER VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.3 NON-VIRAL VECTORS
TABLE 65 MARKET FOR N0N-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 66 MARKET FOR N0N-VIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 67 NORTH AMERICA: MARKET FOR N0N-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 68 EUROPE: MARKET FOR N0N-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 69 ASIA PACIFIC: MARKET FOR N0N-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 70 LATIN AMERICA: MARKET FOR N0N-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
7.3.1 OLIGONUCLEOTIDES
7.3.1.1 Oligonucleotides to account for largest share of non-viral vectors market
TABLE 71 MARKET FOR OLIGONUCLEOTIDES, BY REGION, 2021–2028 (USD MILLION)
TABLE 72 NORTH AMERICA: MARKET FOR OLIGONUCLEOTIDES, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 73 EUROPE: MARKET FOR OLIGONUCLEOTIDES, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 74 ASIA PACIFIC: MARKET FOR OLIGONUCLEOTIDES, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 75 LATIN AMERICA: MARKET FOR OLIGONUCLEOTIDES, BY COUNTRY, 2021–2028 (USD MILLION)
7.3.2 OTHER NON-VIRAL VECTORS
TABLE 76 MARKET FOR OTHER NON-VIRAL VECTORS, BY REGION, 2021–2028 (USD MILLION)
TABLE 77 NORTH AMERICA: MARKET FOR OTHER NON-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 78 EUROPE: MARKET FOR OTHER NON-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 79 ASIA PACIFIC: MARKET FOR OTHER NON-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 80 LATIN AMERICA: MARKET FOR OTHER NON-VIRAL VECTORS, BY COUNTRY, 2021–2028 (USD MILLION)
8 GENE THERAPY MARKET, BY THERAPEUTIC AREA (Page No. - 118)
8.1 INTRODUCTION
TABLE 81 GENE THERAPY INDUSTRY, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
8.2 NEUROLOGY
8.2.1 GROWING NUMBER OF GENE THERAPY PRODUCTS APPROVED FOR NEUROLOGICAL DISEASE TREATMENT TO DRIVE MARKET GROWTH
TABLE 82 CELL AND GENE THERAPY MARKET FOR NEUROLOGY, BY REGION, 2021–2028 (USD MILLION)
TABLE 83 NORTH AMERICA: MARKET FOR NEUROLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 84 EUROPE: MARKET FOR NEUROLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 85 ASIA PACIFIC: MARKET FOR NEUROLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 86 LATIN AMERICA: MARKET FOR NEUROLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
8.3 ONCOLOGY
8.3.1 GROWING DEMAND FOR TARGETED THERAPIES AGAINST CANCERS TO DRIVE MARKET GROWTH
TABLE 87 PIPELINE PRODUCTS FOR ONCOLOGY APPLICATIONS
TABLE 88 NUMBER OF NEW CANCER CASES, BY TYPE, 2020 VS. 2040
TABLE 89 MARKET FOR ONCOLOGY, BY REGION, 2021–2028 (USD MILLION)
TABLE 90 NORTH AMERICA: MARKET FOR ONCOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 91 EUROPE: MARKET FOR ONCOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 92 ASIA PACIFIC: MARKET FOR ONCOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 93 LATIN AMERICA: MARKET FOR ONCOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
8.4 HEPATOLOGY
8.4.1 GROWING R&D ACTIVITIES FOR LIVER CONDITIONS TO SUPPORT GROWTH
TABLE 94 MARKET FOR HEPATOLOGY, BY REGION, 2021–2028 (USD MILLION)
TABLE 95 NORTH AMERICA: MARKET FOR HEPATOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 96 EUROPE: MARKET FOR HEPATOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 97 ASIA PACIFIC: MARKET FOR HEPATOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 98 LATIN AMERICA: MARKET FOR HEPATOLOGY, BY COUNTRY, 2021–2028 (USD MILLION)
8.5 OTHER THERAPEUTIC AREAS
TABLE 99 MARKET FOR OTHER THERAPEUTIC AREAS, BY REGION, 2021–2028 (USD MILLION)
TABLE 100 NORTH AMERICA: MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 101 EUROPE: MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 102 ASIA PACIFIC: MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 103 LATIN AMERICA: MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021–2028 (USD MILLION)
9 GENE THERAPY MARKET, BY DELIVERY METHOD (Page No. - 130)
9.1 INTRODUCTION
TABLE 104 GENE THERAPY INDUSTRY, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
9.2 IN VIVO
9.2.1 IN VIVO DELIVERY METHOD TO ACCOUNT FOR LARGEST MARKET SHARE DURING FORECAST PERIOD
TABLE 105 COMMERCIALIZED AND PIPELINE IN VIVO-BASED GENE THERAPY PRODUCTS
TABLE 106 CELL AND GENE THERAPY MARKET, BY REGION, 2021–2028 (USD MILLION)
TABLE 107 NORTH AMERICA: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 108 EUROPE: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 109 ASIA PACIFIC: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 110 LATIN AMERICA: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
9.3 EX VIVO
9.3.1 GROWING FOCUS ON ONCOLOGY BY MARKET PLAYERS TO SUPPORT MARKET GROWTH
TABLE 111 COMMERCIALIZED AND PIPELINE EX VIVO-BASED GENE THERAPY PRODUCTS
TABLE 112 MARKET, BY REGION, 2021–2028 (USD MILLION)
TABLE 113 NORTH AMERICA: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 114 EUROPE: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 115 ASIA PACIFIC: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 116 LATIN AMERICA: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
10 GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION (Page No. - 138)
10.1 INTRODUCTION
TABLE 117 GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
10.2 INTRAVENOUS
10.2.1 EFFECTIVENESS OF INTRAVENOUS PRODUCTS TO SUPPORT MARKET GROWTH
TABLE 118 CELL AND GENE THERAPY MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY REGION, 2021–2028 (USD MILLION)
TABLE 119 NORTH AMERICA: MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 120 EUROPE: MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 121 ASIA PACIFIC: MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 122 LATIN AMERICA: MARKET FOR INTRAVENOUS ROUTE OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
10.3 OTHER ROUTES OF ADMINISTRATION
TABLE 123 MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY REGION, 2021–2028 (USD MILLION)
TABLE 124 NORTH AMERICA: MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 125 EUROPE: MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 126 ASIA PACIFIC: MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 127 LATIN AMERICA: MARKET FOR OTHER ROUTES OF ADMINISTRATION, BY COUNTRY, 2021–2028 (USD MILLION)
11 GENE THERAPY MARKET, BY REGION (Page No. - 145)
11.1 INTRODUCTION
TABLE 128 GENE THERAPY INDUSTRY, BY REGION, 2021–2028 (USD MILLION)
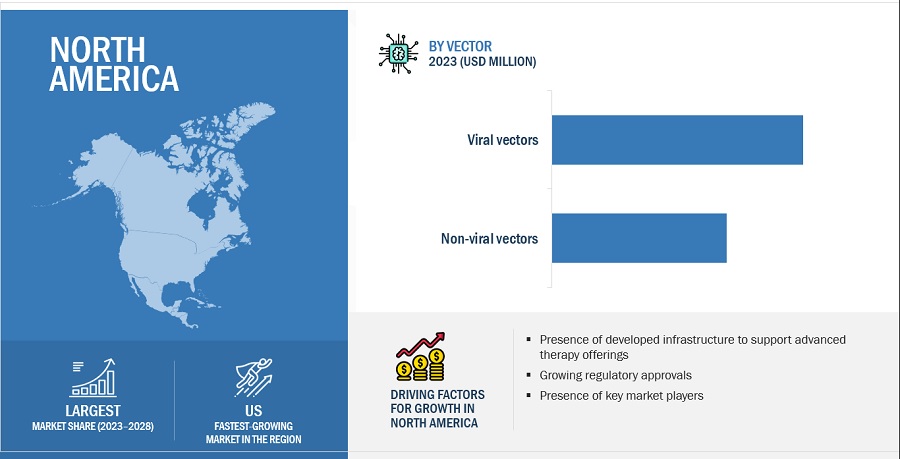
11.2 NORTH AMERICA
FIGURE 28 NORTH AMERICA: GENE THERAPY MARKET SNAPSHOT
TABLE 129 NORTH AMERICA: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 130 NORTH AMERICA: MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 131 NORTH AMERICA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 132 NORTH AMERICA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 133 NORTH AMERICA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 134 NORTH AMERICA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 135 NORTH AMERICA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 136 NORTH AMERICA: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 137 NORTH AMERICA: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.2.1 US
11.2.1.1 Growing regulatory approvals and availability of advanced treatments to drive market growth
TABLE 138 US FDA APPROVALS FOR GENE THERAPY PRODUCTS
TABLE 139 US: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 140 US: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 141 US: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 142 US: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 143 US: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 144 US: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 145 US: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 146 US: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.2.2 CANADA
11.2.2.1 Growing gene therapy research initiatives to support growth
TABLE 147 CANADA: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 148 CANADA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 149 CANADA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 150 CANADA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 151 CANADA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 152 CANADA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 153 CANADA: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 154 CANADA: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.2.3 NORTH AMERICA: RECESSION IMPACT
11.3 EUROPE
FIGURE 29 EUROPE: GENE THERAPY MARKET SNAPSHOT
TABLE 155 EUROPE: MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 156 EUROPE: MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 157 EUROPE: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 158 EUROPE: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 159 EUROPE: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 160 EUROPE: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 161 EUROPE: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 162 EUROPE: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 163 EUROPE: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.1 GERMANY
11.3.1.1 Growing collaborations in market to boost growth
TABLE 164 GERMANY: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 165 GERMANY: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 166 GERMANY: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 167 GERMANY: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 168 GERMANY: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 169 GERMANY: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 170 GERMANY: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 171 GERMANY: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.2 UK
11.3.2.1 Strong government support to drive market growth
TABLE 172 UK: CANCER INCIDENCE, BY TYPE, 2020 VS. 2040
TABLE 173 UK: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 174 UK: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 175 UK: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 176 UK: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 177 UK: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 178 UK: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 179 UK: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 180 UK: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.3 FRANCE
11.3.3.1 Increasing initiatives in genomic research to drive market
TABLE 181 FRANCE: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 182 FRANCE: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 183 FRANCE: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 184 FRANCE: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 185 FRANCE: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 186 FRANCE: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 187 FRANCE: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 188 FRANCE: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.4 ITALY
11.3.4.1 Initiatives for gene therapy development to drive growth
TABLE 189 ITALY: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 190 ITALY: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 191 ITALY: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 192 ITALY: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 193 ITALY: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 194 ITALY: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 195 ITALY: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 196 ITALY: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.5 SPAIN
11.3.5.1 Genomics initiatives and funding to support market growth
TABLE 197 SPAIN: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 198 SPAIN: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 199 SPAIN: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 200 SPAIN: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 201 SPAIN: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 202 SPAIN: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 203 SPAIN: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 204 SPAIN: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.6 REST OF EUROPE
TABLE 205 REST OF EUROPE: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 206 REST OF EUROPE: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 207 REST OF EUROPE: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 208 REST OF EUROPE: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 209 REST OF EUROPE: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 210 REST OF EUROPE: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 211 REST OF EUROPE: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 212 REST OF EUROPE: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.3.7 EUROPE: RECESSION IMPACT
11.4 ASIA PACIFIC
TABLE 213 ASIA PACIFIC: GENE THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 214 ASIA PACIFIC: MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 215 ASIA PACIFIC: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 216 ASIA PACIFIC: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 217 ASIA PACIFIC: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 218 ASIA PACIFIC: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 219 ASIA PACIFIC: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 220 ASIA PACIFIC: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 221 ASIA PACIFIC: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.4.1 CHINA
11.4.1.1 China to dominate gene therapy market in APAC during forecast period
TABLE 222 CHINA: MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 223 CHINA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 224 CHINA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 225 CHINA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 226 CHINA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 227 CHINA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 228 CHINA: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 229 CHINA: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.4.2 JAPAN
11.4.2.1 Increasing initiatives supporting gene therapy adoption to propel market growth
TABLE 230 JAPAN: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 231 JAPAN: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 232 JAPAN: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 233 JAPAN: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 234 JAPAN: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 235 JAPAN: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 236 JAPAN: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 237 JAPAN: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.4.3 INDIA
11.4.3.1 Increasing burden of chronic diseases to drive market growth
TABLE 238 INDIA: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 239 INDIA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 240 INDIA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 241 INDIA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 242 INDIA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 243 INDIA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 244 INDIA: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 245 INDIA: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.4.4 REST OF ASIA PACIFIC
TABLE 246 REST OF ASIA PACIFIC: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 247 REST OF ASIA PACIFIC: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 248 REST OF ASIA PACIFIC: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 249 REST OF ASIA PACIFIC: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 250 REST OF ASIA PACIFIC: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 251 REST OF ASIA PACIFIC: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 252 REST OF ASIA PACIFIC: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 253 REST OF ASIA PACIFIC: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.4.5 ASIA PACIFIC: RECESSION IMPACT
11.5 LATIN AMERICA
TABLE 254 LATIN AMERICA: GENE THERAPY MARKET, BY COUNTRY, 2021–2028 (USD MILLION)
TABLE 255 LATIN AMERICA: MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 256 LATIN AMERICA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 257 LATIN AMERICA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 258 LATIN AMERICA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 259 LATIN AMERICA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 260 LATIN AMERICA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 261 LATIN AMERICA: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 262 LATIN AMERICA: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.5.1 BRAZIL
11.5.1.1 Gradual increase in pharmaceutical R&D to support market growth
TABLE 263 BRAZIL: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 264 BRAZIL: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 265 BRAZIL: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 266 BRAZIL: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 267 BRAZIL: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 268 BRAZIL: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 269 BRAZIL: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 270 BRAZIL: CELL AND GENE THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.5.2 REST OF LATIN AMERICA
TABLE 271 REST OF LATIN AMERICA: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 272 REST OF LATIN AMERICA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 273 REST OF LATIN AMERICA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 274 REST OF LATIN AMERICA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 275 REST OF LATIN AMERICA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 276 REST OF LATIN AMERICA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 277 REST OF LATIN AMERICA: MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 278 REST OF LATIN AMERICA: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.5.3 LATIN AMERICA: RECESSION IMPACT
11.6 MIDDLE EAST
11.6.1 GROWING FOCUS ON GENETIC MEDICINE DEVELOPMENT PROJECTS TO DRIVE MARKET GROWTH IN COMING YEARS
TABLE 279 MIDDLE EAST: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 280 MIDDLE EAST: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 281 MIDDLE EAST: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 282 MIDDLE EAST: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 283 MIDDLE EAST: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 284 MIDDLE EAST: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 285 MIDDLE EAST: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 286 MIDDLE EAST: GENE CELL THERAPY MARKET, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.6.2 MIDDLE EAST: RECESSION IMPACT
11.7 AFRICA
11.7.1 GROWING COLLABORATIONS TO SUPPORT GROWTH IN COMING YEARS
TABLE 287 AFRICA: GENE THERAPY MARKET, BY TYPE, 2021–2028 (USD MILLION)
TABLE 288 AFRICA: MARKET, BY VECTOR, 2021–2028 (USD MILLION)
TABLE 289 AFRICA: MARKET FOR VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 290 AFRICA: MARKET FOR RETROVIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 291 AFRICA: MARKET FOR NON-VIRAL VECTORS, BY TYPE, 2021–2028 (USD MILLION)
TABLE 292 AFRICA: MARKET, BY THERAPEUTIC AREA, 2021–2028 (USD MILLION)
TABLE 293 AFRICA: GENE AND CELL THERAPY MARKET, BY DELIVERY METHOD, 2021–2028 (USD MILLION)
TABLE 294 AFRICA: GENE THERAPY INDUSTRY, BY ROUTE OF ADMINISTRATION, 2021–2028 (USD MILLION)
11.7.2 AFRICA: RECESSION IMPACT
12 COMPETITIVE LANDSCAPE (Page No. - 215)
12.1 KEY PLAYER STRATEGIES
FIGURE 30 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN GENE THERAPY MARKET
12.2 MARKET SHARE ANALYSIS
FIGURE 31 GENE THERAPY INDUSTRY: MARKET SHARE ANALYSIS OF KEY PLAYERS, 2022
TABLE 295 GENE THERAPY INDUSTRY: DEGREE OF COMPETITION
12.3 REVENUE ANALYSIS
FIGURE 32 REVENUE ANALYSIS FOR KEY COMPANIES (2020–2022)
12.4 COMPANY EVALUATION MATRIX
12.4.1 STARS
12.4.2 EMERGING LEADERS
12.4.3 PERVASIVE PLAYERS
12.4.4 PARTICIPANTS
FIGURE 33 COMPANY EVALUATION MATRIX, 2022
12.4.5 COMPANY FOOTPRINT
12.4.5.1 Company therapeutic area footprint
TABLE 296 THERAPEUTIC AREA FOOTPRINT ANALYSIS OF KEY PLAYERS IN GENE THERAPY INDUSTRY
12.4.5.2 Company regional footprint
TABLE 297 REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS IN GENE THERAPY INDUSTRY
12.5 START-UP/SME EVALUATION MATRIX
12.5.1 PROGRESSIVE COMPANIES
12.5.2 RESPONSIVE COMPANIES
12.5.3 DYNAMIC COMPANIES
12.5.4 STARTING BLOCKS
FIGURE 34 START-UP/SME EVALUATION MATRIX, 2022
12.5.5 COMPETITIVE BENCHMARKING
TABLE 298 GENE THERAPY INDUSTRY: DETAILED LIST OF KEY START-UP/SME PLAYERS
TABLE 299 GENE THERAPY INDUSTRY: COMPETITIVE BENCHMARKING OF START-UP/SME PLAYERS
12.6 COMPETITIVE SCENARIO AND TRENDS
12.6.1 PRODUCT APPROVALS
TABLE 300 GENE THERAPY INDUSTRY: PRODUCT APPROVALS, JANUARY 2020–AUGUST 2023
12.6.2 DEALS
TABLE 301 GENE THERAPY INDUSTRY: DEALS, JANUARY 2020–AUGUST 2023
12.6.3 OTHER DEVELOPMENTS
TABLE 302 GENE THERAPY MARKET: OTHER DEVELOPMENTS, JANUARY 2020–AUGUST 2023
13 COMPANY PROFILES (Page No. - 227)
13.1 KEY PLAYERS
(Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))*
13.1.1 NOVARTIS AG
TABLE 303 NOVARTIS AG: COMPANY OVERVIEW
FIGURE 35 NOVARTIS AG: COMPANY SNAPSHOT (2022)
TABLE 304 NOVARTIS AG: PRODUCT APPROVALS
TABLE 305 NOVARTIS AG: DEALS
TABLE 306 NOVARTIS AG: OTHER DEVELOPMENTS
13.1.2 BIOGEN INC.
TABLE 307 BIOGEN INC.: COMPANY OVERVIEW
FIGURE 36 BIOGEN INC.: COMPANY SNAPSHOT (2022)
TABLE 308 BIOGEN INC.: DEALS
TABLE 309 BIOGEN INC.: OTHER DEVELOPMENTS
13.1.3 GILEAD SCIENCES, INC.
TABLE 310 GILEAD SCIENCES, INC.: COMPANY OVERVIEW
FIGURE 37 GILEAD SCIENCES, INC.: COMPANY SNAPSHOT (2022)
TABLE 311 GILEAD SCIENCES, INC.: PRODUCT APPROVALS
TABLE 312 GILEAD SCIENCES, INC.: DEALS
TABLE 313 GILEAD SCIENCES, INC.: OTHER DEVELOPMENTS
13.1.4 BRISTOL-MYERS SQUIBB
TABLE 314 BRISTOL-MYERS SQUIBB: COMPANY OVERVIEW
FIGURE 38 BRISTOL-MYERS SQUIBB: COMPANY SNAPSHOT (2022)
TABLE 315 BRISTOL-MYERS SQUIBB: PRODUCT APPROVALS
TABLE 316 BRISTOL-MYERS SQUIBB: DEALS
13.1.5 ALNYLAM PHARMACEUTICALS, INC.
TABLE 317 ALNYLAM PHARMACEUTICALS, INC.: COMPANY OVERVIEW
FIGURE 39 ALNYLAM PHARMACEUTICALS, INC.: COMPANY SNAPSHOT (2022)
TABLE 318 ALNYLAM PHARMACEUTICALS, INC.: PRODUCT APPROVALS
TABLE 319 ALNYLAM PHARMACEUTICALS, INC.: DEALS
13.1.6 SAREPTA THERAPEUTICS, INC.
TABLE 320 SAREPTA THERAPEUTICS, INC.: COMPANY OVERVIEW
FIGURE 40 SAREPTA THERAPEUTICS: COMPANY SNAPSHOT (2022)
TABLE 321 SAREPTA THERAPEUTICS, INC.: PRODUCT APPROVALS
TABLE 322 SAREPTA THERAPEUTICS, INC.: DEALS
TABLE 323 SAREPTA THERAPEUTICS, INC.: OTHER DEVELOPMENTS
13.1.7 AMGEN, INC.
TABLE 324 AMGEN, INC.: COMPANY OVERVIEW
FIGURE 41 AMGEN, INC.: COMPANY SNAPSHOT (2022)
TABLE 325 AMGEN, INC.: DEALS
TABLE 326 AMGEN, INC.: OTHER DEVELOPMENTS
13.1.8 ORCHARD THERAPEUTICS PLC
TABLE 327 ORCHARD THERAPEUTICS PLC: COMPANY OVERVIEW
FIGURE 42 ORCHARD THERAPEUTICS PLC: COMPANY SNAPSHOT (2022)
TABLE 328 ORCHARD THERAPEUTICS PLC: PRODUCT APPROVALS
TABLE 329 ORCHARD THERAPEUTICS PLC: DEALS
13.1.9 F. HOFFMANN-LA ROCHE AG
TABLE 330 F. HOFFMANN-LA ROCHE AG: COMPANY OVERVIEW
FIGURE 43 F. HOFFMANN-LA ROCHE AG: COMPANY SNAPSHOT (2022)
13.1.10 JAZZ PHARMACEUTICALS PLC
TABLE 331 JAZZ PHARMACEUTICALS PLC: COMPANY OVERVIEW
FIGURE 44 JAZZ PHARMACEUTICALS PLC: COMPANY SNAPSHOT (2022)
TABLE 332 JAZZ PHARMACEUTICALS PLC: DEALS
13.1.11 UNIQURE N.V.
TABLE 333 UNIQURE N.V.: COMPANY OVERVIEW
FIGURE 45 UNIQURE N.V.: COMPANY SNAPSHOT (2022)
TABLE 334 UNIQURE N.V.: PRODUCT APPROVALS
TABLE 335 UNIQURE N.V.: DEALS
13.1.12 JOHNSON & JOHNSON
TABLE 336 JOHNSON & JOHNSON: COMPANY OVERVIEW
FIGURE 46 JOHNSON & JOHNSON: COMPANY SNAPSHOT (2022)
TABLE 337 JOHNSON & JOHNSON: PRODUCT APPROVALS
TABLE 338 JOHNSON & JOHNSON: DEALS
13.1.13 BLUEBIRD BIO, INC.
TABLE 339 BLUEBIRD BIO, INC.: COMPANY OVERVIEW
FIGURE 47 BLUEBIRD BIO, INC.: COMPANY SNAPSHOT (2022)
TABLE 340 BLUEBIRD BIO, INC.: PRODUCT LAUNCHES & APPROVALS
13.1.14 BIOMARIN PHARMACEUTICAL INC.
TABLE 341 BIOMARIN PHARMACEUTICAL INC.: COMPANY OVERVIEW
FIGURE 48 BIOMARIN PHARMACEUTICAL INC.: COMPANY SNAPSHOT (2022)
TABLE 342 BIOMARIN PHARMACEUTICAL INC.: DEALS
TABLE 343 BIOMARIN PHARMACEUTICAL INC.: PRODUCT APPROVALS
13.1.15 KRYSTAL BIOTECH, INC.
TABLE 344 KRYSTAL BIOTECH, INC.: COMPANY OVERVIEW
TABLE 345 KRYSTAL BIOTECH, INC.: PRODUCT APPROVALS
TABLE 346 KRYSTAL BIOTECH, INC.: DEALS
TABLE 347 KRYSTAL BIOTECH, INC.: OTHER DEVELOPMENTS
13.1.16 SHANGHAI SUNWAY BIOTECH CO. LTD.
TABLE 348 SHANGHAI SUNWAY BIOTECH CO. LTD.: COMPANY OVERVIEW
13.1.17 SIBIONO GENETECH CO. LTD.
TABLE 349 SIBIONO GENETECH CO. LTD.: COMPANY OVERVIEW
13.2 OTHER PLAYERS
13.2.1 FERRING B.V.
13.2.2 VERTEX PHARMACEUTICALS INCORPORATED
13.2.3 PFIZER, INC.
13.2.4 SANGAMO THERAPEUTICS, INC.
13.2.5 REGENXBIO
13.2.6 ULTRAGENYX PHARMACEUTICAL INC.
13.2.7 MEIRAGTX HOLDINGS PLC
13.2.8 ANGES, INC.
*Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
14 APPENDIX (Page No. - 281)
14.1 DISCUSSION GUIDE
14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
14.3 CUSTOMIZATION OPTIONS
14.4 RELATED REPORTS
14.5 AUTHOR DETAILS




 Generating Response ...
Generating Response ...







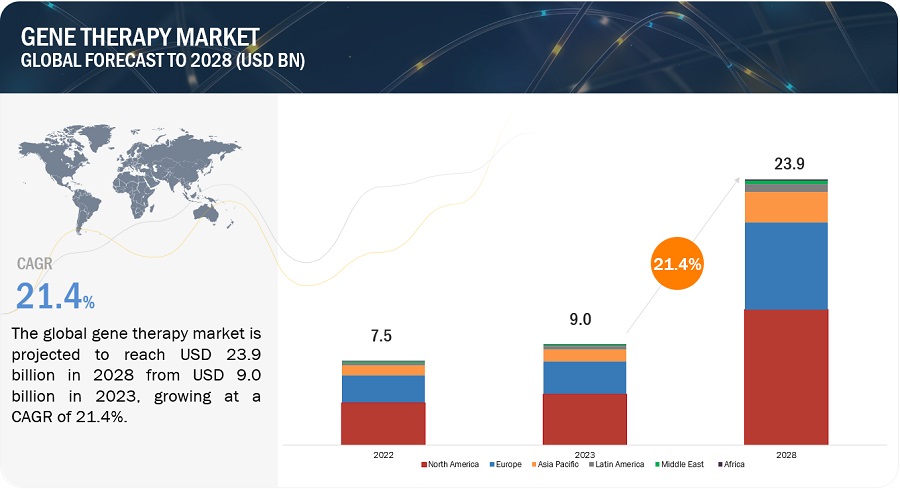
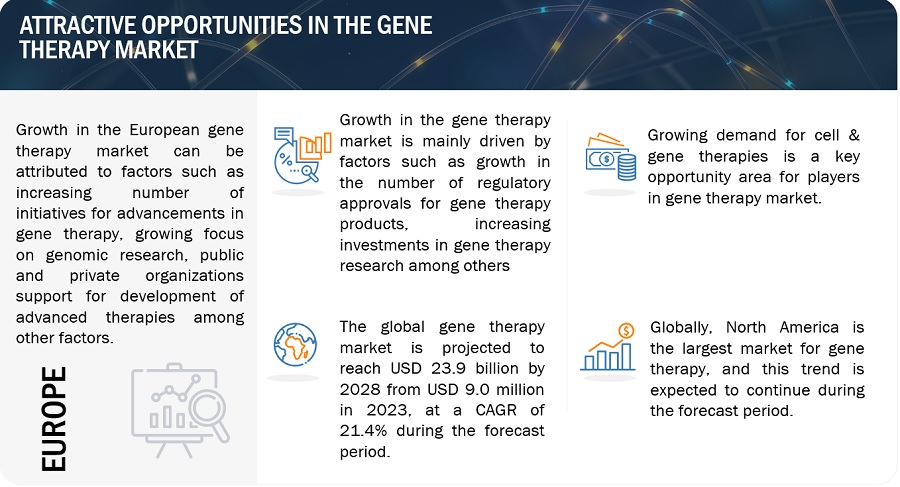
Growth opportunities and latent adjacency in Gene Therapy Market
Can you elaborate on some of the lucrative growth opportunities key players can look forward to in the Gene Therapy Market?
What will be the expected revenue growth by 2029 in the global Gene Therapy Market?