TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 59)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS AND EXCLUSIONS
1.3 MARKET SCOPE
1.3.1 MARKETS COVERED
1.3.2 REGIONS COVERED
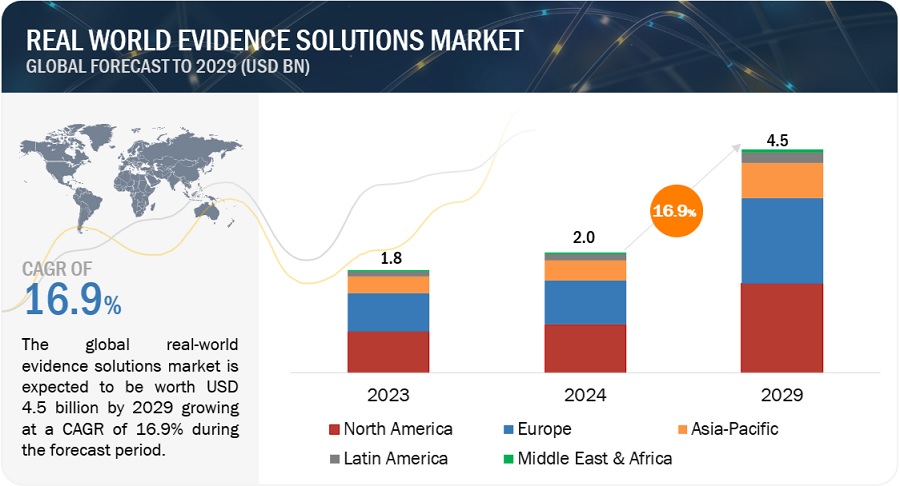
FIGURE 1 REAL-WORLD EVIDENCE SOLUTIONS MARKET
1.3.3 YEARS CONSIDERED
1.3.4 CURRENCY CONSIDERED
1.4 LIMITATIONS
1.5 STAKEHOLDERS
1.6 SUMMARY OF CHANGES
1.7 RECESSION IMPACT
2 RESEARCH METHODOLOGY (Page No. - 65)
2.1 RESEARCH DATA
FIGURE 2 RESEARCH DESIGN
2.1.1 SECONDARY DATA
2.1.1.1 Key data from secondary sources
2.1.2 PRIMARY DATA
FIGURE 3 PRIMARY SOURCES
2.1.2.1 Key data from primary sources
2.1.2.2 Key Industry Insights
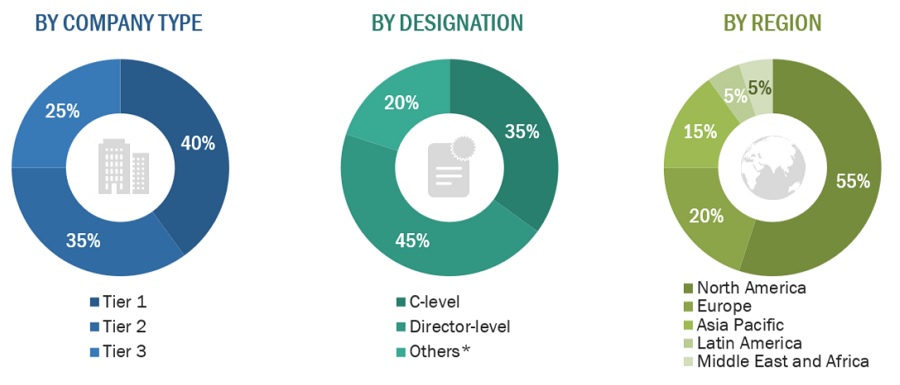
FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
2.2 RESEARCH METHODOLOGY DESIGN
FIGURE 5 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
2.3 MARKET SIZE ESTIMATION
FIGURE 6 SUPPLY-SIDE MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS
2.3.1 BOTTOM-UP APPROACH
FIGURE 7 REAL-WORLD EVIDENCE SOLUTIONS MARKET: BOTTOM-UP APPROACH
2.3.2 TOP-DOWN APPROACH
FIGURE 8 REAL-WORLD EVIDENCE SOLUTIONS MARKET: TOP-DOWN APPROACH
FIGURE 9 CAGR PROJECTIONS FROM ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES IN REAL-WORLD EVIDENCE SOLUTIONS MARKET (2024−2029)
FIGURE 10 REAL-WORLD EVIDENCE SOLUTIONS MARKET: CAGR PROJECTIONS
2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
FIGURE 11 DATA TRIANGULATION METHODOLOGY
2.5 RESEARCH ASSUMPTIONS
2.6 RISK ASSESSMENT
TABLE 1 RISK ASSESSMENT ANALYSIS
2.7 LIMITATIONS
2.7.1 SCOPE-RELATED LIMITATIONS
2.7.2 METHODOLOGY-RELATED LIMITATIONS
2.8 RECESSION IMPACT
3 EXECUTIVE SUMMARY (Page No. - 79)
FIGURE 12 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2024 VS. 2029 (USD MILLION)
FIGURE 13 REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 14 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 15 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2024 VS. 2029 (USD MILLION)
FIGURE 16 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 17 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2024 VS. 2029 (USD MILLION)
FIGURE 18 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2024 VS. 2029 (USD MILLION)
FIGURE 19 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
FIGURE 20 GEOGRAPHICAL SNAPSHOT OF REAL-WORLD EVIDENCE SOLUTIONS MARKET
4 PREMIUM INSIGHTS (Page No. - 85)
4.1 REAL-WORLD EVIDENCE SOLUTIONS MARKET OVERVIEW
FIGURE 21 RISING GERIATRIC POPULATION AND SUBSEQUENT INCREASE IN PREVALENCE OF CHRONIC DISEASES TO DRIVE MARKET
4.2 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER AND COUNTRY (2023)
FIGURE 22 PHARMACEUTICAL & MEDICAL DEVICE COMPANIES ACCOUNTED FOR LARGEST SHARE OF ASIA PACIFIC MARKET IN 2023
4.3 REAL-WORLD EVIDENCE SOLUTIONS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
FIGURE 23 INDIA TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
4.4 REGIONAL MIX: REAL-WORLD EVIDENCE SOLUTIONS MARKET (2024−2029)
FIGURE 24 NORTH AMERICA WILL CONTINUE TO DOMINATE MARKET DURING FORECAST PERIOD
4.5 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DEVELOPED MARKETS VS. EMERGING ECONOMIES
FIGURE 25 EMERGING ECONOMIES TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 89)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 26 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
TABLE 2 MARKET DYNAMICS: IMPACT ANALYSIS
5.2.1 DRIVERS
5.2.1.1 Rising geriatric population and subsequent increase in prevalence of chronic diseases
5.2.1.2 Shift from volume-based care to value-based care
5.2.1.3 Potential of RWE in reducing drug development costs and expediting drug development process
5.2.1.4 Increased R&D spending for development of new pharmaceutical products and medical devices
FIGURE 27 NUMBER OF CLINICAL TRIALS, 2015–2020
5.2.1.5 Support from regulatory bodies for use of RWE solutions
5.2.2 RESTRAINTS
5.2.2.1 Reluctance of medical practitioners and researchers to rely on real-world studies
5.2.3 OPPORTUNITIES
5.2.3.1 Growth opportunities in emerging markets
5.2.3.2 Increased focus on end-to-end RWE services
5.2.4 CHALLENGES
5.2.4.1 Lack of universally accepted methodology standards and data processing infrastructure
5.2.4.2 Shortage of skilled professionals
5.3 INDUSTRY TRENDS
5.3.1 EMERGING ROLE OF WEARABLE DEVICES
5.3.2 SOCIAL MEDIA-SOURCED RWE
5.3.3 RISING USE OF RWD AND RWE ACROSS PHARMACEUTICAL INDUSTRY
FIGURE 28 RECOMMENDED INVESTMENT MODEL FOR RWD AND RWE
5.3.4 INTERNAL VS. OUTSOURCED RWE ANALYTICS APPROACH
FIGURE 29 INTERNAL VS. OUTSOURCED RWE ANALYTICS APPROACH
5.3.5 INCORPORATION OF ARTIFICIAL INTELLIGENCE IN RWD MANAGEMENT
5.4 REAL-WORLD DATA SOURCES
TABLE 3 INDICATIVE LIST OF NATIONAL DATABASES IN DEVELOPED COUNTRIES
5.5 ECOSYSTEM ANALYSIS
FIGURE 30 REAL-WORLD EVIDENCE SOLUTIONS MARKET: ECOSYSTEM
5.6 VALUE CHAIN ANALYSIS
FIGURE 31 REAL-WORLD EVIDENCE SOLUTIONS MARKET: VALUE CHAIN ANALYSIS
5.7 TRENDS/DISRUPTIONS IMPACTING CUSTOMER’S BUSINESS
FIGURE 32 REVENUE SHIFT IN REAL-WORLD EVIDENCE SOLUTIONS MARKET
5.8 PORTER’S FIVE FORCES ANALYSIS
TABLE 4 PORTER’S FIVE FORCES ANALYSIS
5.8.1 THREAT OF NEW ENTRANTS
5.8.2 BARGAINING POWER OF SUPPLIERS
5.8.3 BARGAINING POWER OF BUYERS
5.8.4 THREAT OF SUBSTITUTES
5.8.5 INTENSITY OF COMPETITIVE RIVALRY
5.9 PRICING ANALYSIS
5.9.1 AVERAGE SELLING PRICE, BY KEY PLAYER
5.9.2 INDICATIVE PRICE OF DATA SETS
5.10 TECHNOLOGY ANALYSIS
5.10.1 KEY TECHNOLOGIES
5.10.1.1 Use of AI and ML
5.10.1.2 Blockchain technology
5.10.2 ADJACENT TECHNOLOGIES
5.10.2.1 Predictive analytics
5.10.2.2 Visualization dashboard software
5.11 TARIFF AND REGULATORY LANDSCAPE
5.11.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 5 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.11.2 REGULATORY ANALYSIS
5.11.2.1 North America
5.11.2.1.1 US
5.11.2.1.1.1 Case examples
5.11.2.1.2 Canada
5.11.2.2 Europe
5.11.2.3 Asia Pacific
5.11.2.3.1 China
5.11.2.3.2 Japan
5.11.2.3.3 India
5.12 PATENT ANALYSIS
5.12.1 PATENT PUBLICATION TRENDS FOR RWE SOLUTIONS
FIGURE 33 PATENT PUBLICATION TRENDS IN REAL-WORLD SOLUTIONS MARKET, 2015–2023
5.12.2 JURISDICTION AND TOP APPLICANT ANALYSIS
FIGURE 34 TOP APPLICANTS & OWNERS (COMPANIES/INSTITUTES) FOR REAL-WORLD EVIDENCE SOLUTION PATENTS (JANUARY 2015–DECEMBER 2023)
TABLE 6 LIST OF PATENTS IN REAL-WORLD EVIDENCE SOLUTIONS MARKET, 2019–2023
5.13 KEY CONFERENCES AND EVENTS
TABLE 7 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DETAILED LIST OF CONFERENCES AND EVENTS
5.14 KEY STAKEHOLDERS AND BUYING CRITERIA
5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS
FIGURE 35 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR TOP 3 APPLICATIONS
TABLE 8 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR TOP 3 APPLICATIONS
5.14.2 BUYING CRITERIA
FIGURE 36 KEY BUYING CRITERIA FOR TOP 3 APPLICATIONS
TABLE 9 KEY BUYING CRITERIA FOR TOP 3 APPLICATIONS
5.15 USE CASES/CASE STUDIES
5.15.1 OPTIMIZING RWE WITH SCIENTIFICALLY RIGOROUS DATA-AGNOSTIC APPROACH
5.15.1.1 Use case 1: Designing and building RWE dashboards and leveraging EHR and claims data
5.15.2 UTILIZING DRUG DATA AND RWD TO ENHANCE PATIENT ADHERENCE
5.15.2.1 Use case 2: Integrating specialty pharmacy and patient hub data with RWD data to investigate non-adherence factors
5.15.3 EXISTING ANALYTICS TECHNOLOGY
5.15.3.1 Use case 3: Simplifying data analytics with RWE platform
5.16 END-USER ANALYSIS
5.16.1 UNMET NEEDS
TABLE 10 UNMET NEEDS IN REAL-WORLD EVIDENCE SOLUTIONS MARKET
5.16.2 END-USER EXPECTATIONS
TABLE 11 END-USER EXPECTATIONS IN REAL-WORLD EVIDENCE SOLUTIONS MARKET
5.17 REAL-WORLD EVIDENCE SOLUTIONS BUSINESS MODEL
5.17.1 PLATFORM AS A SERVICE (PAAS) MODEL
5.17.2 DATA PROVIDER MODEL
5.17.3 CONSULTING AND SERVICES MODEL
5.17.4 COLLABORATIVE RESEARCH MODEL
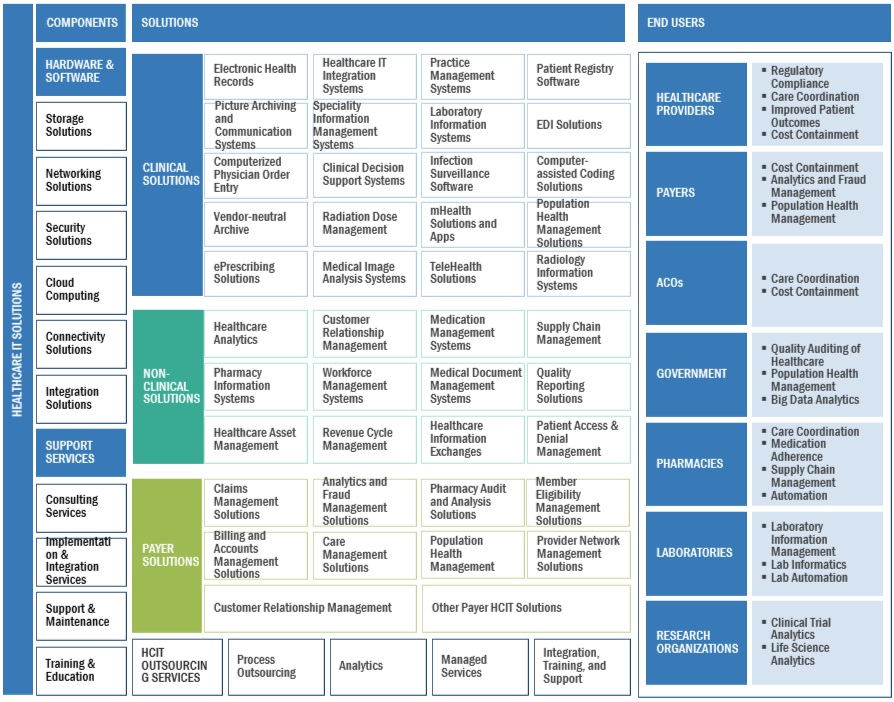
6 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT (Page No. - 125)
6.1 INTRODUCTION
TABLE12 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE13 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
6.2 SERVICES
6.2.1 RISING NEED TO CONVERT DATA INTO ACTIONABLE EVIDENCE TO DRIVE DEMAND
TABLE14 REAL-WORLD EVIDENCE SERVICES OFFERED BY KEY MARKET PLAYERS
TABLE15 REAL-WORLD EVIDENCE SERVICES MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE16 REAL-WORLD EVIDENCE SERVICES MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
6.3 DATA SETS
TABLE17 REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE18 REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE19 REAL-WORLD EVIDENCE DATA SETS MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE20 REAL-WORLD EVIDENCE DATA SETS MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1 DISPARATE DATA SETS
TABLE21 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE22 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE23 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE24 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1.1 Clinical settings data sets
6.3.1.1.1 Increasing utilization of EHR data for trial recruitment to fuel growth
FIGURE 37 ADOPTION OF EHR, BY HOSPITAL SERVICE TYPE, 2019–2021
TABLE 25 CLINICAL SETTINGS DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 26 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL SETTINGS DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 27 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL SETTINGS DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1.2 Claims data sets
6.3.1.2.1 Growing need to understand economic benefits of drug reimbursement by payers to drive growth
TABLE 28 CLAIMS DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 29 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLAIMS DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 30 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLAIMS DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1.3 Pharmacy data sets
6.3.1.3.1 Increasing adoption of e-prescribing systems to drive growth
FIGURE 38 TOTAL E-PRESCRIPTIONS AND CONTROLLED SUBSTANCE PRESCRIPTIONS IN US (2018–2021)
TABLE 31 PHARMACY DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 32 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACY DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 33 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACY DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1.4 Patient-powered data sets
6.3.1.4.1 Increasing need to access opinions on diseases and treatments across social media to drive growth
TABLE 34 PATIENT-POWERED DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 35 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PATIENT-POWERED DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 36 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PATIENT-POWERED DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.1.5 Registry-based data sets
6.3.1.5.1 Increasing number of disease registries to drive demand for registry-based data sets in evidence generation
TABLE 37 USE OF REGISTRIES FOR EVIDENCE GENERATION
TABLE 38 REGISTRY-BASED DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 39 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR REGISTRY-BASED DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 40 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR REGISTRY-BASED DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
6.3.2 INTEGRATED DATA SETS
6.3.2.1 Increasing demand for integrated data from multiple sources to drive growth
TABLE 41 INTEGRATED DATA SETS OFFERED BY KEY MARKET PLAYERS
TABLE 42 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR INTEGRATED DATA SETS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 43 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR INTEGRATED DATA SETS, BY COUNTRY, 2023–2029 (USD MILLION)
7 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION (Page No. - 152)
7.1 INTRODUCTION
FIGURE 39 NUMBER OF CLINICAL TRIALS (COMPLETED), BY CONDITION/DISEASE, SEPTEMBER 2022
TABLE44 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE45 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
7.2 DRUG DEVELOPMENT & APPROVALS
TABLE46 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE47 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE48 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE49 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY COUNTRY, 2023–2029 (USD MILLION)
7.2.1 ONCOLOGY
7.2.1.1 Growing number of clinical trials focused on cancer treatment to drive growth
TABLE 50 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR ONCOLOGY, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 51 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR ONCOLOGY, BY COUNTRY, 2023–2029 (USD MILLION)
7.2.2 CARDIOVASCULAR DISEASE
7.2.2.1 High prevalence of CVD to support market growth
TABLE 52 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 53 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2023–2029 (USD MILLION)
7.2.3 NEUROLOGY
7.2.3.1 Rapidly aging global population and subsequent increase in prevalence of neurological disorders to drive growth
TABLE 54 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR NEUROLOGY, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 55 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR NEUROLOGY, BY COUNTRY, 2023–2029 (USD MILLION)
7.2.4 IMMUNOLOGY
7.2.4.1 Increasing focus on developing innovative products to drive growth
TABLE 56 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR IMMUNOLOGY, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 57 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR IMMUNOLOGY, BY COUNTRY, 2023–2029 (USD MILLION)
7.2.5 OTHER THERAPEUTIC AREAS
TABLE58 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE59 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2023–2029 (USD MILLION)
7.3 MEDICAL DEVICE DEVELOPMENT & APPROVALS
7.3.1 INCREASING RESEARCH IN MEDICAL DEVICE DEVELOPMENT TO DRIVE DEMAND
TABLE60 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR MEDICAL DEVICE DEVELOPMENT & APPROVALS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE61 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR MEDICAL DEVICE DEVELOPMENT & APPROVALS, BY COUNTRY, 2023–2029 (USD MILLION)
7.4 POST-MARKET SURVEILLANCE
7.4.1 EXTENSIVE USE OF RWE SOLUTIONS IN POST-MARKET SURVEILLANCE TO BOOST MARKET
TABLE62 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR POST-MARKET SURVEILLANCE, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE63 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR POST-MARKET SURVEILLANCE, BY COUNTRY, 2023–2029 (USD MILLION)
7.5 MARKET ACCESS & REIMBURSEMENT/COVERAGE DECISION-MAKING
7.5.1 GROWING USE OF RWE SOLUTIONS TO DEVELOP ECONOMIC AND BUDGET IMPACT MODELS TO FAVOR MARKET GROWTH
TABLE64 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR MARKET ACCESS & REIMBURSEMENT/COVERAGE DECISION-MAKING, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE65 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR MARKET ACCESS & REIMBURSEMENT/COVERAGE DECISION-MAKING, BY COUNTRY, 2023–2029 (USD MILLION)
7.6 CLINICAL & REGULATORY DECISION-MAKING
7.6.1 LIMITED VALIDITY OF RANDOMIZED CONTROLLED TRIALS TO DRIVE USE OF RWE SOLUTIONS
TABLE66 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL & REGULATORY DECISION-MAKING, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE67 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL & REGULATORY DECISION-MAKING, BY COUNTRY, 2023–2029 (USD MILLION)
8 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL (Page No. - 175)
8.1 INTRODUCTION
TABLE68 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE69 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
8.2 PAY PER USE (VALUE-BASED PRICING)
8.2.1 RISING DEMAND FOR COST-EFFECTIVE AND FLEXIBLE SOLUTIONS TO BOOST MARKET
TABLE70 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PAY-PER-USE (VALUE-BASED PRICING) MODELS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE71 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PAY-PER-USE (VALUE-BASED PRICING) MODELS, BY COUNTRY, 2023–2029 (USD MILLION)
8.3 SUBSCRIPTION
8.3.1 RISING PREFERENCE FOR FLEXIBILITY AND SCALABILITY TO SUPPORT MARKET GROWTH
TABLE72 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR SUBSCRIPTION MODELS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE73 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR SUBSCRIPTION MODELS, BY COUNTRY, 2023–2029 (USD MILLION)
9 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE (Page No. - 181)
9.1 INTRODUCTION
TABLE 74 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE75 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
9.2 ON-PREMISE
9.2.1 ENHANCED DATA CONTROL BENEFITS TO BOOST DEMAND
TABLE76 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR ON-PREMISE MODELS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE77 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR ON-PREMISE MODELS, BY COUNTRY, 2023–2029 (USD MILLION)
9.3 CLOUD-BASED
9.3.1 INCREASED SCALABILITY AND FLEXIBILITY IN DATA COLLECTION TO DRIVE GROWTH
TABLE78 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE79 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR CLOUD-BASED MODELS, BY COUNTRY, 2023–2029 (USD MILLION)
10 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER (Page No. - 187)
10.1 INTRODUCTION
TABLE 80 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 81 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
10.2 PHARMACEUTICAL & MEDICAL DEVICE COMPANIES
10.2.1 INCREASED R&D EXPENDITURE IN INNOVATIVE MEDICINES TO DRIVE MARKET GROWTH
TABLE 82 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACEUTICAL & MEDICAL DEVICE COMPANIES, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 83 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACEUTICAL & MEDICAL DEVICE COMPANIES, BY COUNTRY, 2023–2029 (USD MILLION)
10.3 HEALTHCARE PAYERS
10.3.1 INCREASED FOCUS ON OUTCOME-BASED PAYMENT MODELS TO DRIVE DEMAND
TABLE 84 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PAYERS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 85 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PAYERS, BY COUNTRY, 2023–2029 (USD MILLION)
10.4 HEALTHCARE PROVIDERS
10.4.1 GROWING FOCUS ON IMPROVING PROFITABILITY TO SUPPORT MARKET GROWTH
TABLE 86 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PROVIDERS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 87 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PROVIDERS, BY COUNTRY, 2023–2029 (USD MILLION)
10.5 OTHER END USERS
TABLE 88 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER END USERS, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 89 REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2029 (USD MILLION)
11 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REGION (Page No. - 198)
11.1 INTRODUCTION
TABLE 90 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2018–2022 (USD MILLION)
TABLE 91 REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023–2029 (USD MILLION)
11.2 NORTH AMERICA
11.2.1 NORTH AMERICA: RECESSION IMPACT
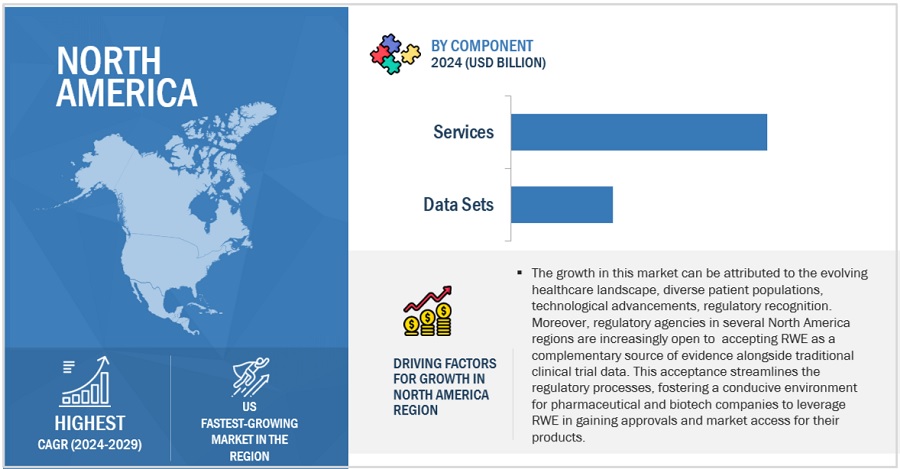
FIGURE 40 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET SNAPSHOT
TABLE 92 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 93 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
TABLE 94 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 95 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 96 NORTH AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 97 NORTH AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 98 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 99 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 100 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 101 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 102 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 103 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 104 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 105 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 106 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 107 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 108 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 109 NORTH AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.2.2 US
11.2.2.1 US to hold largest share of North American market
TABLE 110 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 111 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 112 US: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 113 US: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 114 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 115 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 116 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 117 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 118 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 119 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 120 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 121 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 122 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 123 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 124 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 125 US: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.2.3 CANADA
11.2.3.1 Rising number of clinical trials in Canada to drive market
TABLE 126 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 127 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 128 CANADA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 129 CANADA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 130 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 131 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 132 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 133 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 134 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 135 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 136 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 137 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 138 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 139 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 140 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 141 CANADA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3 EUROPE
11.3.1 EUROPE: RECESSION IMPACT
TABLE 142 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 143 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
TABLE 144 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 145 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 146 EUROPE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 147 EUROPE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 148 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 149 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 150 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 151 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 152 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 153 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 154 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 155 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 156 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 157 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 158 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 159 EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.2 GERMANY
11.3.2.1 High pharmaceutical R&D spending in Germany to boost market growth
TABLE 160 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 161 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 162 GERMANY: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 163 GERMANY: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 164 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 165 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 166 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 167 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 168 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 169 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 170 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 171 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 172 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 173 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 174 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 175 GERMANY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.3 UK
11.3.3.1 Growing adoption of HTA to support market growth
TABLE 176 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 177 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 178 UK: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 179 UK: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 180 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 181 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 182 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 183 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 184 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 185 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 186 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 187 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 188 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 189 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 190 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 191 UK: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.4 FRANCE
11.3.4.1 High number of oncology clinical trials in France to drive market growth
TABLE 192 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 193 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 194 FRANCE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 195 FRANCE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 196 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 197 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 198 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 199 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 200 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 201 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 202 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 203 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 204 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 205 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 206 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 207 FRANCE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.5 ITALY
11.3.5.1 High demand for RWE due to widespread use of pay-for-outcomes to drive market
TABLE 208 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 209 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 210 ITALY: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 211 ITALY: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 212 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 213 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 214 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 215 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 216 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 217 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 218 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 219 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 220 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 221 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 222 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 223 ITALY: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.6 SPAIN
11.3.6.1 Rising R&D expenditure to propel market growth in Spain
TABLE 224 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 225 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 226 SPAIN: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 227 SPAIN: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 228 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 229 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 230 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 231 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 232 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 233 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 234 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 235 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 236 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 237 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 238 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 239 SPAIN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.3.7 REST OF EUROPE
TABLE 240 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 241 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 242 REST OF EUROPE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 243 REST OF EUROPE: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 244 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 245 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 246 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 247 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 248 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 249 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 250 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 251 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 252 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 253 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 254 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 255 REST OF EUROPE: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.4 ASIA PACIFIC
11.4.1 ASIA PACIFIC: RECESSION IMPACT
FIGURE 41 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET SNAPSHOT
TABLE 256 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 257 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
TABLE 258 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 259 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 260 ASIA PACIFIC: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 261 ASIA PACIFIC: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 262 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 263 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 264 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 265 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 266 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 267 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 268 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 269 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 270 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 271 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 272 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 273 ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.4.2 JAPAN
11.4.2.1 Stringent regulatory scenario in Japan to restrain market growth
TABLE 274 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 275 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 276 JAPAN: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 277 JAPAN: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 278 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 279 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 280 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 281 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 282 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 283 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 284 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 285 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 286 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 287 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 288 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 289 JAPAN: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.4.3 CHINA
11.4.3.1 Low cost of clinical trials and large pharmaceutical R&D base in China to drive market
TABLE 290 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 291 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 292 CHINA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 293 CHINA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 294 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 295 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 296 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 297 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 298 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 299 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 300 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 301 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 302 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 303 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 304 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 305 CHINA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.4.4 INDIA
11.4.4.1 Growing adoption of outcome-based research to drive market
TABLE 306 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 307 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 308 INDIA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 309 INDIA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 310 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 311 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 312 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 313 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 314 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 315 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 316 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 317 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 318 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 319 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 320 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 321 INDIA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.4.5 REST OF ASIA PACIFIC
TABLE 322 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 323 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 324 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 325 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 326 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 327 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 328 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 329 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 330 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 331 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 332 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 333 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 334 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 335 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 336 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 337 REST OF ASIA PACIFIC: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.5 LATIN AMERICA
11.5.1 LATIN AMERICA: RECESSION IMPACT
TABLE 338 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2018–2022 (USD MILLION)
TABLE 339 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2023–2029 (USD MILLION)
TABLE 340 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 341 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 342 LATIN AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 343 LATIN AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 344 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 345 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 346 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 347 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 348 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 349 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 350 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 351 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 352 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 353 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 354 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 355 LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.5.2 BRAZIL
11.5.2.1 Brazil to hold largest share of LATAM market
TABLE 356 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 357 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 358 BRAZIL: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 359 BRAZIL: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 360 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 361 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 362 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 363 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 364 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 365 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 366 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 367 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 368 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 369 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 370 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 371 BRAZIL: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.5.3 MEXICO
11.5.3.1 Rising funding and investment in pharma R&D to favor growth
TABLE 372 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 373 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 374 MEXICO: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 375 MEXICO: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 376 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 377 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 378 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 379 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 380 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 381 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 382 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 383 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 384 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 385 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 386 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 387 MEXICO: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.5.4 REST OF LATIN AMERICA
TABLE 388 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 389 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 390 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 391 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 392 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 393 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 394 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 395 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 396 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 397 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 398 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 399 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 400 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 401 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 402 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 403 REST OF LATIN AMERICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.6 MIDDLE EAST & AFRICA
11.6.1 MIDDLE EAST & AFRICA: RECESSION IMPACT
TABLE 404 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2018–2022 (USD MILLION)
TABLE 405 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023–2029 (USD MILLION)
TABLE 406 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 407 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 408 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 409 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 410 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 411 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 412 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 413 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 414 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 415 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 416 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 417 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 418 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 419 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 420 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 421 MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.6.2 GCC COUNTRIES
11.6.2.1 Growing availability of healthcare funding to offer opportunities for market growth
TABLE 422 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 423 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 424 GCC COUNTRIES: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 425 GCC COUNTRIES: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 426 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 427 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 428 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 429 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 430 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 431 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 432 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 433 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 434 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 435 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 436 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 437 GCC COUNTRIES: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
11.6.3 REST OF MIDDLE EAST & AFRICA
TABLE 438 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2018–2022 (USD MILLION)
TABLE 439 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023–2029 (USD MILLION)
TABLE 440 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2018–2022 (USD MILLION)
TABLE 441 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023–2029 (USD MILLION)
TABLE 442 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2018–2022 (USD MILLION)
TABLE 443 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023–2029 (USD MILLION)
TABLE 444 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2018–2022 (USD MILLION)
TABLE 445 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023–2029 (USD MILLION)
TABLE 446 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2018–2022 (USD MILLION)
TABLE 447 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023–2029 (USD MILLION)
TABLE 448 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2018–2022 (USD MILLION)
TABLE 449 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023–2029 (USD MILLION)
TABLE 450 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2018–2022 (USD MILLION)
TABLE 451 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023–2029 (USD MILLION)
TABLE 452 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2018–2022 (USD MILLION)
TABLE 453 REST OF MIDDLE EAST & AFRICA: REAL-WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023–2029 (USD MILLION)
12 COMPETITIVE LANDSCAPE (Page No. - 351)
12.1 INTRODUCTION
12.2 RIGHT-TO-WIN APPROACHES ADOPTED BY KEY PLAYERS
TABLE 454 REAL-WORLD EVIDENCE SOLUTIONS MARKET: STRATEGIES ADOPTED
12.3 REVENUE SHARE ANALYSIS
FIGURE 42 REVENUE ANALYSIS OF KEY PLAYERS (2018–2022)
12.4 MARKET SHARE ANALYSIS
FIGURE 43 MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
TABLE 455 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DEGREE OF COMPETITION
12.5 MARKET RANKING ANALYSIS
FIGURE 44 REAL-WORLD EVIDENCE SOLUTIONS MARKET RANKING, BY KEY PLAYER, 2023
12.6 BRAND/PRODUCT COMPARATIVE ANALYSIS
FIGURE 45 BRAND/PRODUCT COMPARATIVE ANALYSIS, BY KEY PLAYER
12.6.1 IQVIA HOLDINGS INC. (US)
12.6.2 MERATIVE (US)
12.6.3 ICON PLC (IRELAND)
12.6.4 FLATIRON HEALTH (US)
12.6.5 VERANTOS (US)
12.6.6 TRINETX, LLC (US)
12.7 VALUATION AND FINANCIAL METRICS OF REAL-WORLD EVIDENCE SOLUTIONS VENDORS
FIGURE 46 EV/EBITDA OF KEY VENDORS
12.8 COMPANY EVALUATION MATRIX: KEY PLAYERS
12.8.1 STARS
12.8.2 EMERGING LEADERS
12.8.3 PERVASIVE PLAYERS
12.8.4 PARTICIPANTS
FIGURE 47 REAL-WORLD EVIDENCE SOLUTIONS MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
12.8.5 COMPANY FOOTPRINT
FIGURE 48 REAL-WORLD EVIDENCE SOLUTIONS MARKET: COMPANY FOOTPRINT
TABLE 456 REAL-WORLD EVIDENCE SOLUTIONS MARKET: COMPONENT FOOTPRINT
TABLE 457 REAL-WORLD EVIDENCE SOLUTIONS MARKET: END-USER FOOTPRINT
TABLE 458 REAL-WORLD EVIDENCE SOLUTIONS MARKET: REGIONAL FOOTPRINT
12.9 COMPANY EVALUATION MATRIX: START-UPS/SMES
12.9.1 PROGRESSIVE COMPANIES
12.9.2 RESPONSIVE COMPANIES
12.9.3 DYNAMIC COMPANIES
12.9.4 STARTING BLOCKS
FIGURE 49 REAL-WORLD EVIDENCE SOLUTIONS MARKET: COMPANY EVALUATION MATRIX (START-UPS/SMES), 2023
12.9.5 COMPETITIVE BENCHMARKING
TABLE 459 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DETAILED LIST OF KEY START-UPS/SMES
TABLE 460 REAL-WORLD EVIDENCE SOLUTIONS MARKET: COMPETITIVE BENCHMARKING OF KEY START-UPS/SMES
12.1 COMPETITIVE SCENARIO
12.10.1 PRODUCT LAUNCHES/ENHANCEMENTS
TABLE 461 REAL-WORLD EVIDENCE SOLUTIONS MARKET: PRODUCT LAUNCHES/ENHANCEMENTS, JANUARY 2020–DECEMBER 2023
12.10.2 DEALS
TABLE 462 REAL-WORLD EVIDENCE SOLUTIONS MARKET: DEALS, JANUARY 2020–DECEMBER 2023
13 COMPANY PROFILES (Page No. - 371)
(Business Overview, Products/Solutions/Services Offered, Recent Developments, MnM view (Key strengths/Right to win, Strategic choices made, Weakness/competitive threats)*
13.1 KEY PLAYERS
13.1.1 IQVIA HOLDINGS INC.
TABLE 463 IQVIA HOLDINGS INC.: COMPANY OVERVIEW
FIGURE 50 IQVIA HOLDINGS INC.: COMPANY SNAPSHOT (2022)
TABLE 464 IQVIA HOLDINGS INC: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2020–DECEMBER 2023
TABLE 465 IQVIA HOLDINGS INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.2 MERATIVE
TABLE 466 MERATIVE: COMPANY OVERVIEW
13.1.2.2 Products/Services/Solutions offered
13.1.2.3 Recent developments
13.1.2.3.1 Deals
TABLE 467 MERATIVE: DEALS, JANUARY 2020–DECEMBER 2023
13.1.3 OPTUM, INC.
TABLE 468 OPTUM, INC.: COMPANY OVERVIEW
FIGURE 51 OPTUM, INC.: COMPANY SNAPSHOT (2022)
TABLE 469 OPTUM, INC.: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2020–DECEMBER 2023
13.1.4 ICON PLC
TABLE 470 ICON PLC: COMPANY OVERVIEW
FIGURE 52 ICON PLC: COMPANY SNAPSHOT (2022)
TABLE 471 ICON PLC: DEALS, JANUARY 2020–DECEMBER 2023
13.1.5 SYNEOS HEALTH, INC.
TABLE 472 SYNEOS HEALTH INC.: COMPANY OVERVIEW
FIGURE 53 SYNEOS HEALTH INC.: COMPANY SNAPSHOT (2022)
TABLE 473 SYNEOS HEALTH, INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.6 PAREXEL INTERNATIONAL CORPORATION
TABLE 474 PAREXEL INTERNATIONAL CORPORATION: COMPANY OVERVIEW
TABLE 475 PAREXEL INTERNATIONAL CORPORATION: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2020–DECEMBER 2023
TABLE 476 PAREXEL INTERNATIONAL CORPORATION: DEALS, JANUARY 2020–DECEMBER 2023
13.1.7 THERMO FISHER SCIENTIFIC INC.
TABLE 477 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
FIGURE 54 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2022)
13.1.7.2 Products/Services/Solutions offered
13.1.7.3 Recent developments
TABLE 478 THERMO FISHER SCIENTIFIC INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.8 FORTREA INC.
TABLE 479 FORTREA INC.: COMPANY OVERVIEW
13.1.9 ORACLE CORPORATION
TABLE 480 ORACLE CORPORATION: COMPANY OVERVIEW
FIGURE 55 ORACLE CORPORATION: COMPANY SNAPSHOT (2022)
TABLE 481 ORACLE CORPORATION: DEALS, JANUARY 2020–DECEMBER 2023
13.1.10 ELEVANCE HEALTH, INC.
TABLE 482 ELEVANCE HEALTH, INC.: COMPANY OVERVIEW
FIGURE 56 ELEVANCE HEALTH, INC.: COMPANY SNAPSHOT (2022)
TABLE 483 ELEVANCE HEALTH, INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.11 SAS INSTITUTE INC.
TABLE 484 SAS INSTITUTE INC.: COMPANY OVERVIEW
TABLE 485 SAS INSTITUTE INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.12 AETION, INC.
TABLE 486 AETION, INC.: COMPANY OVERVIEW
TABLE 487 AETION, INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.13 TRINETX LLC
TABLE 488 TRINETX LLC: COMPANY OVERVIEW
TABLE 489 TRINETX LLC: PRODUCT LAUNCHES, JANUARY 2020–DECEMBER 2023
TABLE 490 TRINETX LLC: DEALS, JANUARY 2020–DECEMBER 2023
13.1.14 TRINITY
TABLE 491 TRINITY: COMPANY OVERVIEW
TABLE 492 TRINITY: DEALS, JANUARY 2020–DECEMBER 2023
13.1.15 PERKINELMER INC.
TABLE 493 PERKINELMER INC.: COMPANY OVERVIEW
FIGURE 57 PERKINELMER INC.: COMPANY SNAPSHOT (2022)
TABLE 494 PERKINELMER INC.: PRODUCT LAUNCHES, JANUARY 2020–DECEMBER 2023
13.1.16 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION
TABLE 495 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION: COMPANY OVERVIEW
FIGURE 58 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION: COMPANY SNAPSHOT (2022)
13.1.17 CEGEDIM HEALTH DATA
TABLE 496 CEGEDIM HEALTH DATA: COMPANY OVERVIEW
TABLE 497 CEGEDIM HEALTH DATA: DEALS, JANUARY 2020–DECEMBER 2023
13.1.18 VERANTOS
TABLE 498 VERANTOS: COMPANY OVERVIEW
TABLE 499 VERANTOS INC.: DEALS, JANUARY 2020–DECEMBER 2023
13.1.19 MEDPACE HOLDINGS, INC.
TABLE 500 MEDPACE HOLDINGS, INC.: COMPANY OVERVIEW
FIGURE 59 MEDPACE HOLDINGS, INC.: COMPANY SNAPSHOT (2022)
13.2 OTHER PLAYERS
13.2.1 HEALTHVERITY, INC.
TABLE 501 HEALTHVERITY, INC.: COMPANY OVERVIEW
13.2.2 DATAVANT
TABLE 502 DATAVANT: COMPANY OVERVIEW
13.2.3 SYAPSE, INC.
TABLE 503 SYAPSE, INC.: COMPANY OVERVIEW
13.2.4 TEMPUS
TABLE 504 TEMPUS: COMPANY OVERVIEW
13.2.5 FLATIRON HEALTH
TABLE 505 FLATIRON HEALTH: COMPANY OVERVIEW
13.2.6 QUANTZIG
TABLE 506 QUANTZIG: COMPANY OVERVIEW
*Details on Business Overview, Products/Solutions/Services Offered, Recent Developments, MnM view (Key strengths/Right to win, Strategic choices made, Weakness/competitive threats)* might not be captured in case of unlisted companies.
14 APPENDIX (Page No. - 423)
14.1 DISCUSSION GUIDE
14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
14.3 CUSTOMIZATION OPTIONS
14.4 RELATED REPORTS
14.5 AUTHOR DETAILS






 Generating Response ...
Generating Response ...







Growth opportunities and latent adjacency in Real World Evidence Solutions Market
Which are the different countries covered across the regions of the Global RWE Solutions Market?
Which geographical segment holds the major share of the global RWE Solutions Market?
What are the benefits of the emerging trends in the global RWE Solutions Market?