TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 51)
1.1 STUDY OBJECTIVES
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS AND EXCLUSIONS
1.3 MARKET SCOPE
1.3.1 MARKETS COVERED
1.3.2 REGIONS COVERED
1.3.3 YEARS CONSIDERED
1.3.4 CURRENCY CONSIDERED
1.4 STAKEHOLDERS
1.5 SUMMARY OF CHANGES
1.5.1 RECESSION IMPACT
2 RESEARCH METHODOLOGY (Page No. - 56)
2.1 RESEARCH DATA
2.2 RESEARCH APPROACH
FIGURE 1 RESEARCH DESIGN
2.2.1 SECONDARY DATA
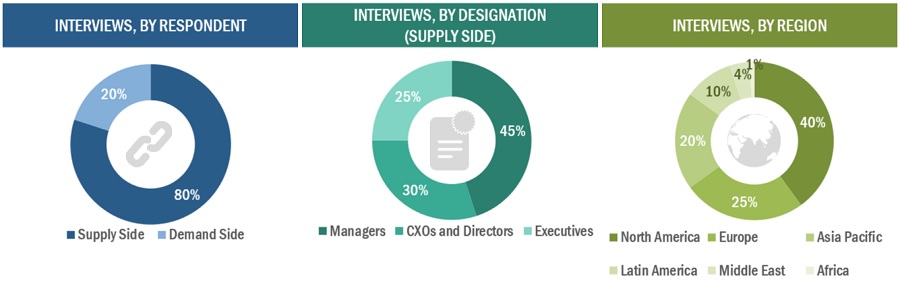
2.2.2 PRIMARY DATA
FIGURE 2 BREAKDOWN OF PRIMARIES
2.2.2.1 Primary research
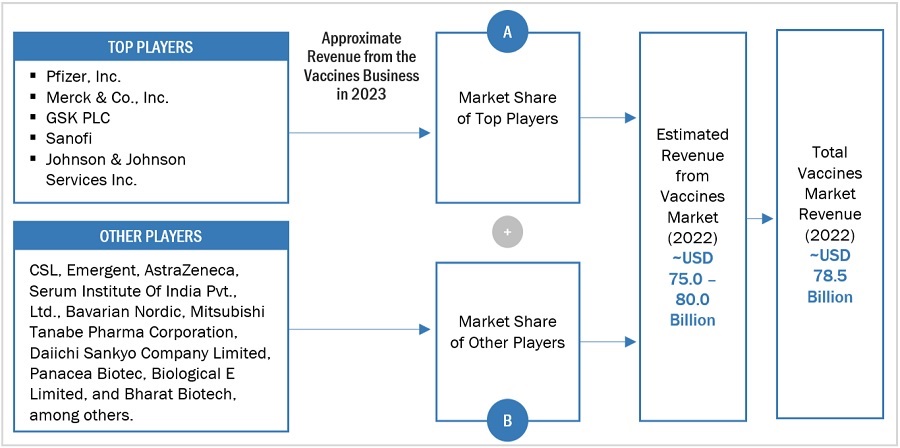
2.3 MARKET SIZE ESTIMATION a
FIGURE 3 MARKET SIZE ESTIMATION (SUPPLY SIDE ANALYSIS), 2023
FIGURE 4 MARKET SIZE ESTIMATION: APPROACH 1 (REVENUE SHARE ANALYSIS), 2023
FIGURE 5 ILLUSTRATIVE EXAMPLE OF PFIZER INC.: REVENUE SHARE ANALYSIS, 2023
2.3.1 INSIGHTS FROM PRIMARIES
FIGURE 6 MARKET VALIDATION FROM PRIMARY EXPERTS
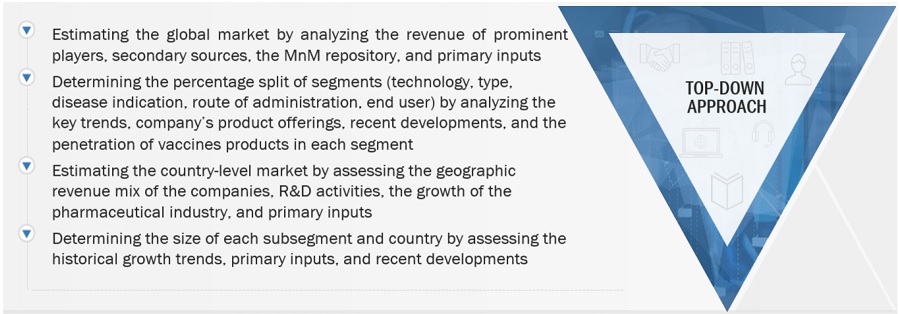
2.3.2 SEGMENTAL ASSESSMENT
FIGURE 7 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
2.4 GROWTH RATE ASSUMPTIONS
FIGURE 8 VACCINES MARKET: CAGR PROJECTION (2024-2029)
FIGURE 9 VACCINES INDUSTRY: GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, CHALLENGES, AND OPPORTUNITIES
2.5 MARKET BREAKDOWN AND DATA TRIANGULATION
FIGURE 10 DATA TRIANGULATION METHODOLOGY
2.6 STUDY ASSUMPTIONS
2.7 RESEARCH LIMITATIONS
2.8 RISK ASSESSMENT
2.9 RECESSION IMPACT ANALYSIS
3 EXECUTIVE SUMMARY (Page No. - 69)
FIGURE 11 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2024 VS. 2029 (USD MILLION)
FIGURE 12 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2024 VS. 2029 (USD MILLION)
FIGURE 13 MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 14 MARKET (INCLUDING COVID-19 VACCINES), BY TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 15 MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2024 VS. 2029 (USD MILLION)
FIGURE 16 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2024 VS. 2029 (USD MILLION)
FIGURE 17 MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2024 VS. 2029 (USD MILLION)
FIGURE 18 MARKET (INCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2024 VS. 2029 (USD MILLION)
FIGURE 19 MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2024 VS. 2029 (USD MILLION)
FIGURE 20 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY END USER, 2024 VS. 2029 (USD MILLION)
FIGURE 21 GEOGRAPHICAL SNAPSHOT OF MARKET (EXCLUDING COVID-19 VACCINES)
FIGURE 22 GEOGRAPHICAL SNAPSHOT OF MARKET (INCLUDING COVID-19 VACCINES)
4 PREMIUM INSIGHTS (Page No. - 81)
4.1 VACCINES MARKET OVERVIEW (INCLUDING COVID-19 VACCINES)
FIGURE 23 RISING PREVALENCE OF INFECTIOUS DISEASES TO DRIVE MARKET
4.2 VACCINES INDUSTRY OVERVIEW (EXCLUDING COVID-19 VACCINES)
FIGURE 24 LAUNCH OF RSV VACCINES AND STRONG PRODUCT PIPELINE TO DRIVE MARKET
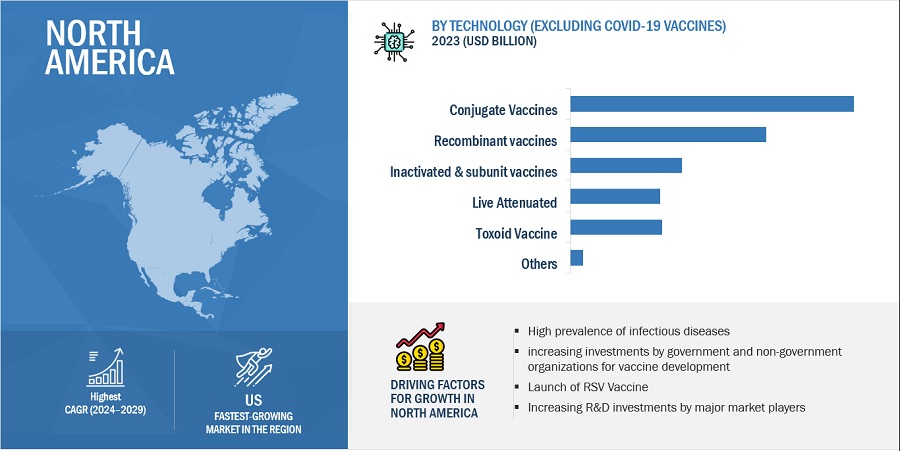
4.3 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY AND COUNTRY, 2023
FIGURE 25 US AND CONJUGATE VACCINES ACCOUNTED FOR LARGEST SHARE OF NORTH AMERICAN MARKET IN 2023
4.4 GEOGRAPHICAL GROWTH OPPORTUNITIES: MARKET (EXCLUDING COVID-19 VACCINES)
FIGURE 26 ASIA PACIFIC MARKETS TO REGISTER HIGHEST GROWTH RATES DURING FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 85)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 27 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES: VACCINES MARKET
TABLE 1 IMPACT ANALYSIS: VACCINES INDUSTRY
5.2.1 DRIVERS
5.2.1.1 Focus on vaccine development and launches
5.2.1.2 Rising prevalence of infectious diseases
FIGURE 28 US: INCIDENCE OF TUBERCULOSIS, 2017–2021
5.2.1.3 Increasing immunization programs
5.2.1.4 Advancements in vaccine technology
5.2.1.5 Government support and funding for vaccine development
TABLE 2 NIH FUNDING FOR VACCINE RESEARCH, 2020–2024 (USD MILLION)
5.2.2 RESTRAINTS
5.2.2.1 High cost of vaccine development
5.2.3 OPPORTUNITIES
5.2.3.1 Rising focus on therapeutic vaccines
5.2.3.2 Extensive R&D for vaccines and increased investments in clinical trials
5.2.4 CHALLENGES
5.2.4.1 Stringent regulatory processes
5.2.4.2 Product recalls
5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS’ BUSINESSES
FIGURE 29 REVENUE SHIFTS AND NEW REVENUE POCKETS FOR VACCINE PROVIDERS
5.4 PRICING ANALYSIS
5.4.1 AVERAGE SELLING PRICE OF PRODUCTS, BY KEY PLAYER
TABLE 3 AVERAGE SELLING PRICE OF PEDIATRIC VACCINES
TABLE 4 AVERAGE SELLING PRICE OF ADULT VACCINES
5.4.2 AVERAGE SELLING PRICE, BY PRODUCT TYPE
TABLE 5 AVERAGE SELLING PRICE OF CONJUGATE VACCINES
TABLE 6 AVERAGE SELLING PRICE OF RECOMBINANT VACCINES
TABLE 7 AVERAGE SELLING PRICE OF INACTIVATED & SUBUNIT VACCINES
TABLE 8 AVERAGE SELLING PRICE OF LIVE ATTENUATED VACCINES
TABLE 9 AVERAGE SELLING PRICE OF TOXOID VACCINES
TABLE 10 AVERAGE SELLING PRICE OF M-RNA VACCINES
5.4.3 AVERAGE SELLING PRICE TREND
5.5 TECHNOLOGY ANALYSIS
5.5.1 MRNA VACCINES
5.5.2 DNA TECHNOLOGY
5.5.3 VLP VACCINE TECHNOLOGY
5.6 VALUE CHAIN ANALYSIS
FIGURE 30 VALUE CHAIN ANALYSIS: RAW MATERIAL AND MANUFACTURING PHASES TO CONTRIBUTE MAXIMUM VALUE
5.7 PIPELINE ANALYSIS
FIGURE 31 VACCINES MARKET: CLINICAL TRIALS, BY PHASE
FIGURE 32 VACCINES INDUSTRY: CLINICAL TRIALS, BY DISEASE INDICATION
TABLE 11 PIPELINE PRODUCTS UNDER PHASE 2 AND PHASE 3 CLINICAL TRIALS
TABLE 12 VACCINE PIPELINE PRODUCTS UNDER PHASE-3 CLINICAL TRIALS FOR RSV
5.7.1 KEY PIPELINE PRODUCTS
TABLE 13 KEY PIPELINE VACCINES: GSK PLC
TABLE 14 KEY PIPELINE VACCINES: MERCK & CO., INC.
TABLE 15 KEY PIPELINE VACCINES: PFIZER INC.
TABLE 16 KEY PIPELINE VACCINES: SANOFI S.A.
5.8 ECOSYSTEM/MARKET MAP
FIGURE 33 ECOSYSTEM/MARKET MAP
TABLE 17 ROLE IN ECOSYSTEM: MARKET
5.9 REGULATORY ANALYSIS
TABLE 18 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 19 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 20 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 21 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 22 MIDDLE EAST: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 23 AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.10 PORTER’S FIVE FORCES ANALYSIS
TABLE 24 PORTER’S FIVE FORCES ANALYSIS
5.10.1 THREAT OF NEW ENTRANTS
5.10.2 THREAT OF SUBSTITUTES
5.10.3 BARGAINING POWER OF SUPPLIERS
5.10.4 BARGAINING POWER OF BUYERS
5.10.5 INTENSITY OF COMPETITIVE RIVALRY
5.11 PATENT ANALYSIS
FIGURE 34 PATENT APPLICATIONS FOR VACCINES, SEPTEMBER 2013–SEPTEMBER 2023
TABLE 25 MARKET: INDICATIVE LIST OF PATENTS
5.12 KEY CONFERENCES & EVENTS, 2024–2025
TABLE 26 DETAILED LIST OF CONFERENCES & EVENTS
5.13 KEY STAKEHOLDERS & BUYING CRITERIA
5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
FIGURE 35 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF VACCINES
5.13.2 KEY BUYING CRITERIA
FIGURE 36 KEY BUYING CRITERIA FOR END USERS
6 VACCINES MARKET, BY TECHNOLOGY (Page No. - 124)
6.1 INTRODUCTION
TABLE 27 VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 28 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
6.2 CONJUGATE VACCINES
6.2.1 INCREASING PUBLIC-PRIVATE PARTNERSHIPS TO DRIVE MARKET
TABLE 29 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 30 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 31 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 32 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 33 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.3 RECOMBINANT VACCINES
6.3.1 LOW POST-VACCINATION REACTIONS AND REDUCED NEED FOR BOOSTER DOSES TO DRIVE MARKET
TABLE 34 EXAMPLES OF RECOMBINANT VACCINES
TABLE 35 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 36 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 37 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 38 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 39 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.4 INACTIVATED & SUBUNIT VACCINES
6.4.1 EASE OF STORAGE AND TRANSPORTATION TO SUPPORT GROWTH
TABLE 40 EXAMPLES OF INACTIVATED & SUBUNIT VACCINES
TABLE 41 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 42 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 43 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 44 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 45 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.5 LIVE ATTENUATED VACCINES
6.5.1 HIGH COST OF STORAGE AND LIMITED FINANCIAL RESOURCES OF DISTRIBUTORS TO RESTRAIN MARKET
TABLE 46 EXAMPLES OF LIVE ATTENUATED VACCINES
TABLE 47 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 48 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 49 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 50 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 51 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.6 TOXOID VACCINES
6.6.1 RISING PREVALENCE OF BACTERIAL INFECTIONS AMONG INFANTS AND CHILDREN TO DRIVE MARKET
TABLE 52 EXAMPLES OF TOXOID VACCINES
TABLE 53 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 54 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 55 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 56 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 57 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.7 VIRAL VECTOR VACCINES
6.7.1 RISING INVESTMENT IN VACCINE DEVELOPMENT TO DRIVE MARKET
TABLE 58 MARKET (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 59 NORTH AMERICA: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 60 EUROPE: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 61 ASIA PACIFIC: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 62 LATIN AMERICA: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.8 MRNA VACCINES
6.8.1 INCREASING FOCUS ON MRNA VACCINE DEVELOPMENT TO DRIVE MARKET
TABLE 63 MARKET (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 64 NORTH AMERICA: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 65 EUROPE: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 66 ASIA PACIFIC: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 67 LATIN AMERICA: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
6.9 OTHER VACCINES
TABLE 68 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 69 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 70 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 71 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 72 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
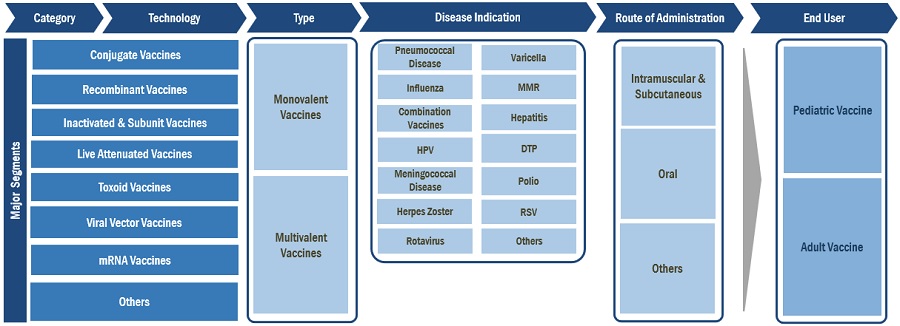
7 VACCINES MARKET, BY TYPE (Page No. - 154)
7.1 INTRODUCTION
TABLE 73 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 74 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
7.2 MULTIVALENT VACCINES
7.2.1 INCREASED NEED FOR IMMUNIZATION AND COST-EFFECTIVENESS TO DRIVE MARKET
TABLE 75 EXAMPLES OF MULTIVALENT VACCINES
TABLE 76 MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 77 NORTH AMERICA: MULTIVALENT VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 78 EUROPE: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 79 ASIA PACIFIC: MULTIVALENT VACCINES INDUSTRY(EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 80 LATIN AMERICA: MULTIVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
7.3 MONOVALENT VACCINES
7.3.1 RISING R&D INVESTMENTS AND PREVALENCE OF INFECTIOUS DISEASES TO SUPPORT MARKET GROWTH
TABLE 81 EXAMPLES OF MONOVALENT VACCINES
TABLE 82 MONOVALENT VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 83 NORTH AMERICA: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 84 EUROPE: MONOVALENT VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 85 ASIA PACIFIC: MONOVALENT VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 86 LATIN AMERICA: MONOVALENT VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8 VACCINES MARKET, BY DISEASE INDICATION (Page No. - 165)
8.1 INTRODUCTION
TABLE 87 VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 88 MARKET (INCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
8.2 PNEUMOCOCCAL DISEASE
8.2.1 INCREASING INCIDENCE OF PNEUMONIA IN CHILDREN TO DRIVE MARKET
TABLE 89 LIST OF COMMERCIALLY AVAILABLE PNEUMOCOCCAL DISEASE VACCINES
TABLE 90 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 91 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 92 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 93 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 94 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.3 INFLUENZA
8.3.1 RISING NEED FOR IMMUNIZATION AGAINST VIRAL INFECTIONS TO DRIVE MARKET
TABLE 95 LIST OF COMMERCIALLY AVAILABLE INFLUENZA VACCINES
TABLE 96 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 97 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 98 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 99 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 100 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.4 COMBINATION VACCINES
8.4.1 GROWING DEMAND FOR ALL-IN-ONE VACCINE TO DRIVE MARKET
TABLE 101 LIST OF COMMERCIALLY AVAILABLE COMBINATION VACCINES
TABLE 102 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 103 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 104 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 105 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 106 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.5 HPV
8.5.1 GROWING INCIDENCE OF SEXUALLY TRANSMITTED DISEASES TO DRIVE MARKET
TABLE 107 LIST OF COMMERCIALLY AVAILABLE HPV VACCINES
TABLE 108 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 109 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 110 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 111 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 112 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.6 MENINGOCOCCAL DISEASE
8.6.1 INCREASING INITIATIVES BY GOVERNMENT AND NON-GOVERNMENT ORGANIZATIONS TO DRIVE MARKET
TABLE 113 LIST OF COMMERCIALLY AVAILABLE MENINGOCOCCAL DISEASE VACCINES
TABLE 114 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 115 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 116 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 117 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 118 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.7 HERPES ZOSTER
8.7.1 INCREASING INVESTMENT IN NOVEL VACCINE DEVELOPMENT TO DRIVE MARKET
TABLE 119 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 120 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 121 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 122 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 123 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.8 ROTAVIRUS
8.8.1 GROWING FOCUS OF GOVERNMENT AND NON-GOVERNMENT BODIES ON ROTAVIRUS PREVENTION TO PROPEL MARKET
TABLE 124 LIST OF COMMERCIALLY AVAILABLE ROTAVIRUS VACCINES
TABLE 125 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 126 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 127 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 128 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 129 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.9 MMR
8.9.1 RISING INCIDENCE OF MEASLES, MUMPS, AND RUBELLA TO BOOST DEMAND
TABLE 130 LIST OF COMMERCIALLY AVAILABLE MMR VACCINES
TABLE 131 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 132 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 133 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 134 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 135 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.10 VARICELLA
8.10.1 INCREASING PROMOTION OF IMMUNIZATION PROGRAMS TO SUPPORT MARKET GROWTH
TABLE 136 LIST OF COMMERCIALLY AVAILABLE VARICELLA VACCINES
TABLE 137 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 138 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 139 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 140 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 141 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.11 HEPATITIS
8.11.1 LOW SOCIO-ECONOMIC STANDARDS OF LIVING AND HIGH CONTAMINATION IN DRINKING WATER TO DRIVE MARKET
TABLE 142 LIST OF COMMERCIALLY AVAILABLE HEPATITIS VACCINES
TABLE 143 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 144 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 145 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 146 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 147 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.12 DTP
8.12.1 INCREASED OCCURRENCE OF DIPHTHERIA, TETANUS, AND PERTUSSIS IN EMERGING ECONOMIES TO DRIVE MARKET
TABLE 148 LIST OF COMMERCIALLY AVAILABLE DTP VACCINES
TABLE 149 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 150 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 151 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 152 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 153 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.13 POLIO
8.13.1 INCREASING GOVERNMENT INITIATIVES AND IMMUNIZATION PROGRAMS TO DRIVE MARKET
TABLE 154 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 155 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 156 EUROPE: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 157 ASIA PACIFIC: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 158 LATIN AMERICA: MARKET (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.14 RSV
8.14.1 STRONG PRODUCT PIPELINE AND NEW PRODUCT LAUNCHES TO PROPEL MARKET GROWTH
TABLE 159 MARKET (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 160 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 161 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 162 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 163 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
8.15 OTHER DISEASE INDICATIONS
FIGURE 37 US: NUMBER OF COVID-19 CASES, 2020–2023
TABLE 164 LIST OF COMMERCIALLY AVAILABLE VACCINES FOR OTHER DISEASE INDICATIONS
TABLE 165 MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 166 MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 167 NORTH AMERICA: MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 168 NORTH AMERICA: MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 169 EUROPE: MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 170 EUROPE: MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 171 ASIA PACIFIC: MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 172 ASIA PACIFIC: MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 173 LATIN AMERICA: MARKET FOR OTHER DISEASE INDICATIONS (INCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 174 LATIN AMERICA: MARKET FOR OTHER DISEASE INDICATIONS (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
9 VACCINES MARKET, BY ROUTE OF ADMINISTRATION (Page No. - 221)
9.1 INTRODUCTION
TABLE 175 VACCINES INDUSTRY, BY ROUTE OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), 2022–2029 (USD MILLION)
TABLE 176 MARKET, BY ROUTE OF ADMINISTRATION (INCLUDING COVID-19 VACCINES), 2022–2029 (USD MILLION)
9.2 INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION
9.2.1 EASE OF ABSORPTION AND BETTER IMMUNE RESPONSE TO DRIVE ADOPTION
TABLE 177 ADVANTAGES AND DISADVANTAGES OF INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION
TABLE 178 MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 179 NORTH AMERICA: MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 180 EUROPE: MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 181 ASIA PACIFIC: MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 182 LATIN AMERICA: MARKET FOR INTRAMUSCULAR & SUBCUTANEOUS ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
9.3 ORAL ADMINISTRATION
9.3.1 REDUCED RISK OF BLOOD-TRANSMITTED INFECTIONS TO DRIVE ADOPTION
TABLE 183 MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 184 NORTH AMERICA: MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 185 EUROPE: MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 186 ASIA PACIFIC: MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 187 LATIN AMERICA: MARKET FOR ORAL ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
9.4 OTHER ROUTES OF ADMINISTRATION
TABLE 188 VACCINES INDUSTRY FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 189 NORTH AMERICA: VACCINES INDUSTRY FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 190 EUROPE: VACCINES INDUSTRY FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 191 ASIA PACIFIC: VACCINES INDUSTRY FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 192 LATIN AMERICA: VACCINES INDUSTRY FOR OTHER ROUTES OF ADMINISTRATION (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
10 VACCINES MARKET, BY END USER (Page No. - 234)
10.1 INTRODUCTION
TABLE 193 MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
TABLE 194 MARKET (INCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
10.2 ADULT VACCINES
10.2.1 ADULT VACCINES TO COMMAND LARGER MARKET SHARE
TABLE 195 ADULT VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 196 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 197 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 198 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 199 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
10.3 PEDIATRIC VACCINES
10.3.1 SUPPORT FROM GOVERNMENT AND NON-GOVERNMENT BODIES TO DRIVE MARKET
TABLE 200 PEDIATRIC VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 201 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 202 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 203 ASIA PACIFIC: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 204 LATIN AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
11 VACCINES MARKET, BY REGION (Page No. - 243)
11.1 INTRODUCTION
TABLE 205 VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
TABLE 206 VACCINES INDUSTRY (INCLUDING COVID-19 VACCINES), BY REGION, 2022–2029 (USD MILLION)
11.2 NORTH AMERICA
11.2.1 NORTH AMERICA: RECESSION IMPACT
FIGURE 38 NORTH AMERICA: VACCINES MARKET SNAPSHOT (EXCLUDING COVID-19 VACCINES)
TABLE 207 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 208 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 209 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 210 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 211 NORTH AMERICA: MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 212 NORTH AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.2.2 US
11.2.2.1 US to dominate North American market
TABLE 213 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 214 US: MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 215 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 216 US: MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 217 US: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.2.3 CANADA
11.2.3.1 High incidence of infectious diseases to drive market
TABLE 218 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 219 CANADA: MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 220 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 221 CANADA: MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 222 CANADA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3 EUROPE
11.3.1 EUROPE: RECESSION IMPACT
TABLE 223 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 224 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 225 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 226 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 227 EUROPE: MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 228 EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.2 GERMANY
11.3.2.1 Significant R&D investments and growing biotechnology industry to drive market
TABLE 229 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 230 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 231 GERMANY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 232 GERMANY: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.3 UK
11.3.3.1 Launch of new products and increased funding by government and non-government organizations to drive market
TABLE 233 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 234 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 235 UK: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 236 UK: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.4 FRANCE
11.3.4.1 Favorable government initiatives for mass immunization to drive market
TABLE 237 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 238 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 239 FRANCE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 240 FRANCE: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.5 ITALY
11.3.5.1 Higher investments by companies for increased production capacities to drive market
TABLE 241 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 242 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 243 ITALY: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 244 ITALY: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.6 SPAIN
11.3.6.1 Rising investments in vaccine development by private organizations to drive market
TABLE 245 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 246 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 247 SPAIN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 248 SPAIN: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.3.7 REST OF EUROPE
TABLE 249 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 250 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 251 REST OF EUROPE: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 252 REST OF EUROPE: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4 ASIA PACIFIC
11.4.1 ASIA PACIFIC: RECESSION IMPACT
FIGURE 39 ASIA PACIFIC: VACCINES MARKET SNAPSHOT (EXCLUDING COVID-19 VACCINES)
TABLE 253 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 254 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 255 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 256 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 257 ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 258 ASIA PACIFIC: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4.2 JAPAN
11.4.2.1 Favorable government initiatives to support market growth
TABLE 259 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 260 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 261 JAPAN: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 262 JAPAN: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4.3 SOUTH KOREA
11.4.3.1 Strong government strategies for improved vaccine hubs to drive market
TABLE 263 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 264 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 265 SOUTH KOREA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 266 SOUTH KOREA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4.4 CHINA
11.4.4.1 China to hold largest share in APAC vaccines market
TABLE 267 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 268 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 269 CHINA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 270 CHINA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4.5 INDIA
11.4.5.1 Increasing government initiatives and development of new and improved vaccines to drive market
TABLE 271 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 272 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 273 INDIA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 274 INDIA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.4.6 REST OF ASIA PACIFIC
TABLE 275 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 276 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 277 REST OF ASIA PACIFIC: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 278 REST OF ASIA PACIFIC: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.5 LATIN AMERICA
11.5.1 LATIN AMERICA: RECESSION IMPACT
TABLE 279 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 280 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 281 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 282 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 283 LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 284 LATIN AMERICA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.5.2 BRAZIL
11.5.2.1 Rising focus on immunization programs to drive market
TABLE 285 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 286 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 287 BRAZIL: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 288 BRAZIL: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.5.3 MEXICO
11.5.3.1 Trained workforce and ethnically varied population base for clinical trials to propel market growth
TABLE 289 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 290 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 291 MEXICO: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 292 MEXICO: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.5.4 REST OF LATIN AMERICA
TABLE 293 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 294 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 295 REST OF LATIN AMERICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 296 REST OF LATIN AMERICA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.6 MIDDLE EAST & AFRICA
11.6.1 MIDDLE EAST & AFRICA: RECESSION IMPACT
11.6.2 MIDDLE EAST
11.6.2.1 Increasing prevalence of infectious diseases to drive market
TABLE 297 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 298 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 299 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY DISEASE INDICATION, 2022–2029 (USD MILLION)
TABLE 300 MIDDLE EAST: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 301 MIDDLE EAST: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
11.6.3 AFRICA
11.6.3.1 Availability of funds and grants from developed economies to drive market
TABLE 302 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TECHNOLOGY, 2022–2029 (USD MILLION)
TABLE 303 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY TYPE, 2022–2029 (USD MILLION)
TABLE 304 AFRICA: VACCINES MARKET (EXCLUDING COVID-19 VACCINES), BY ROUTE OF ADMINISTRATION, 2022–2029 (USD MILLION)
TABLE 305 AFRICA: VACCINES INDUSTRY (EXCLUDING COVID-19 VACCINES), BY END USER, 2022–2029 (USD MILLION)
12 COMPETITIVE LANDSCAPE (Page No. - 309)
12.1 INTRODUCTION
12.2 KEY STRATEGIES/RIGHT TO WIN
FIGURE 40 STRATEGIES ADOPTED BY MAJOR PLAYERS IN VACCINES MARKET
12.3 REVENUE SHARE ANALYSIS
FIGURE 41 REVENUE SHARE ANALYSIS, 2021–2023
12.4 MARKET SHARE ANALYSIS
FIGURE 42 MARKET SHARE ANALYSIS OF KEY PLAYERS (2023)
TABLE 306 VACCINES MARKET: DEGREE OF COMPETITION
12.5 COMPANY EVALUATION MATRIX: KEY PLAYERS
12.5.1 STARS
12.5.2 EMERGING LEADERS
12.5.3 PERVASIVE PLAYERS
12.5.4 PARTICIPANTS
FIGURE 43 VACCINES MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
12.5.5 COMPANY FOOTPRINT
TABLE 307 TYPE FOOTPRINT
TABLE 308 REGIONAL FOOTPRINT
12.6 COMPANY EVALUATION MATRIX: START-UP/SME PLAYERS
12.6.1 PROGRESSIVE COMPANIES
12.6.2 RESPONSIVE COMPANIES
12.6.3 DYNAMIC COMPANIES
12.6.4 STARTING BLOCKS
FIGURE 44 VACCINES MARKET: COMPANY EVALUATION MATRIX (START-UP/SME PLAYERS), 2023
12.6.5 COMPETITIVE BENCHMARKING OF START-UPS/SMES
TABLE 309 VACCINES MARKET: DETAILED LIST OF KEY START-UPS/SMES
TABLE 310 VACCINES MARKET: COMPETITIVE BENCHMARKING OF START-UPS/SMES
12.7 COMPETITIVE SCENARIOS
12.7.1 PRODUCT LAUNCHES & APPROVALS
TABLE 311 VACCINES MARKET: PRODUCT LAUNCHES & APPROVALS (JANUARY 2021−APRIL 2024)
12.7.2 DEALS
TABLE 312 VACCINES MARKET: DEALS (JANUARY 2021−APRIL 2024)
12.7.3 EXPANSIONS
TABLE 313 VACCINES MARKET: EXPANSIONS (JANUARY 2021−APRIL 2024)
12.7.4 OTHER DEVELOPMENTS
TABLE 314 VACCINES MARKET: OTHER DEVELOPMENTS (JANUARY 2021−APRIL 2024)
13 COMPANY PROFILES (Page No. - 325)
(Business Overview, Products Offered, Recent Developments, MnM View Right to win, Strategic choices made, Weaknesses and competitive threats) *
13.1 KEY PLAYERS
13.1.1 GSK PLC
TABLE 315 GSK PLC: COMPANY OVERVIEW
FIGURE 45 GSK PLC: COMPANY SNAPSHOT (2023)
TABLE 316 GSK PLC: PRODUCTS OFFERED
TABLE 317 GSK PLC: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 318 GSK PLC: DEALS (JANUARY 2020–MARCH 2024)
TABLE 319 GSK PLC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.2 MERCK & CO., INC.
TABLE 320 MERCK & CO., INC.: COMPANY OVERVIEW
FIGURE 46 MERCK & CO., INC.: COMPANY SNAPSHOT (2023)
TABLE 321 MERCK & CO., INC.: PRODUCTS OFFERED
TABLE 322 MERCK & CO., INC.: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 323 MERCK & CO., INC.: DEALS (JANUARY 2020–MARCH 2024)
TABLE 324 MERCK & CO., INC.: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.3 PFIZER INC.
TABLE 325 PFIZER INC.: COMPANY OVERVIEW
FIGURE 47 PFIZER INC.: COMPANY SNAPSHOT (2023)
TABLE 326 PFIZER INC.: PRODUCTS OFFERED
TABLE 327 PFIZER INC.: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 328 PFIZER INC.: DEALS (JANUARY 2020–MARCH 2024)
TABLE 329 PFIZER INC.: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.4 SANOFI
TABLE 330 SANOFI: COMPANY OVERVIEW
FIGURE 48 SANOFI: COMPANY SNAPSHOT (2023)
TABLE 331 SANOFI: PRODUCTS OFFERED
TABLE 332 SANOFI: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 333 SANOFI: DEALS (JANUARY 2020–MARCH 2024)
TABLE 334 SANOFI: EXPANSIONS (JANUARY 2020–MARCH 2024)
TABLE 335 SANOFI: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.5 CSL
TABLE 336 CSL: COMPANY OVERVIEW
FIGURE 49 CSL: COMPANY SNAPSHOT (2023)
TABLE 337 CSL: PRODUCTS OFFERED
TABLE 338 CSL: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 339 CSL: DEALS (JANUARY 2020–MARCH 2024)
TABLE 340 CSL: EXPANSIONS (JANUARY 2020–MARCH 2024)
TABLE 341 CSL: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.6 EMERGENT
TABLE 342 EMERGENT: COMPANY OVERVIEW
FIGURE 50 EMERGENT: COMPANY SNAPSHOT (2023)
TABLE 343 EMERGENT: PRODUCTS OFFERED
TABLE 344 EMERGENT: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 345 EMERGENT: DEALS (JANUARY 2020–MARCH 2024)
TABLE 346 EMERGENT: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.7 JOHNSON & JOHNSON SERVICES, INC.
TABLE 347 JOHNSON & JOHNSON SERVICES INC.: COMPANY OVERVIEW
FIGURE 51 JOHNSON & JOHNSON SERVICES INC.: COMPANY SNAPSHOT (2023)
TABLE 348 JOHNSON & JOHNSON SERVICES INC.: PRODUCTS OFFERED
TABLE 349 JOHNSON & JOHNSON SERVICES INC.: PRODUCT APPROVALS (JANUARY 2020− MARCH 2024)
TABLE 350 JOHNSON & JOHNSON SERVICES INC.: DEALS (JANUARY 2020–MARCH 2024)
13.1.8 ASTRAZENECA
TABLE 351 ASTRAZENECA: COMPANY OVERVIEW
FIGURE 52 ASTRAZENECA: COMPANY SNAPSHOT (2023)
TABLE 352 ASTRAZENECA: PRODUCTS OFFERED
TABLE 353 ASTRAZENECA: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 354 ASTRAZENECA: DEALS (JANUARY 2020–MARCH 2024)
TABLE 355 ASTRAZENECA: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.9 SERUM INSTITUTE OF INDIA PVT., LTD.
TABLE 356 SERUM INSTITUTE OF INDIA PVT., LTD.: COMPANY OVERVIEW
TABLE 357 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS OFFERED (SUPPLIED OVERSEAS)
TABLE 358 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS OFFERED (SUPPLIED IN INDIA)
TABLE 359 SERUM INSTITUTE OF INDIA PVT., LTD.: PRODUCTS LAUNCHES & APPROVALS
TABLE 360 SERUM INSTITUTE OF INDIA PVT., LTD.: DEALS (JANUARY 2020–MARCH 2024)
13.1.10 BAVARIAN NORDIC
TABLE 361 BAVARIAN NORDIC: COMPANY OVERVIEW
FIGURE 53 BAVARIAN NORDIC: COMPANY SNAPSHOT (2023)
TABLE 362 BAVARIAN NORDIC: PRODUCTS OFFERED
TABLE 363 BAVARIAN NORDIC: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 364 BAVARIAN NORDIC: DEALS (JANUARY 2020–MARCH 2024)
TABLE 365 BAVARIAN NORDIC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.11 MITSUBISHI TANABE PHARMA CORPORATION
TABLE 366 MITSUBISHI TANABE PHARMA CORPORATION: COMPANY OVERVIEW
TABLE 367 MITSUBISHI TANABE PHARMA CORPORATION: PRODUCTS OFFERED
TABLE 368 MITSUBISHI TANABE PHARMA CORPORATION: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 369 MITSUBISHI TANABE PHARMA CORPORATION: DEALS (JANUARY 2020–MARCH 2024)
13.1.12 DAIICHI SANKYO COMPANY, LIMITED
TABLE 370 DAIICHI SANKYO COMPANY, LIMITED: COMPANY OVERVIEW
FIGURE 54 DAIICHI SANKYO COMPANY, LIMITED: COMPANY SNAPSHOT (2022)
TABLE 371 DAIICHI SANKYO COMPANY, LIMITED: PRODUCTS OFFERED
TABLE 372 DAIICHI SANKYO COMPANY, LIMITED: DEALS (JANUARY 2020–MARCH 2024)
13.1.13 PANACEA BIOTEC
TABLE 373 PANACEA BIOTEC: COMPANY OVERVIEW
FIGURE 55 PANACEA BIOTEC: COMPANY SNAPSHOT (2022)
TABLE 374 PANACEA BIOTEC: PRODUCTS OFFERED
TABLE 375 PANACEA BIOTEC: DEALS (JANUARY 2020–MARCH 2024)
TABLE 376 PANACEA BIOTEC: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.14 BIOLOGICAL E LIMITED
TABLE 377 BIOLOGICAL E LIMITED: COMPANY OVERVIEW
TABLE 378 BIOLOGICAL E LIMITED: PRODUCTS OFFERED (INDIAN MARKET)
TABLE 379 BIOLOGICAL E LIMITED: PRODUCTS OFFERED (INTERNATIONAL MARKET)
TABLE 380 BIOLOGICAL E LIMITED: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 381 BIOLOGICAL E LIMITED: DEALS (JANUARY 2020–MARCH 2024)
TABLE 382 BIOLOGICAL E LIMITED: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.1.15 BHARAT BIOTECH
TABLE 383 BHARAT BIOTECH: COMPANY OVERVIEW
TABLE 384 BHARAT BIOTECH: PRODUCTS OFFERED (VACCINES)
TABLE 385 BHARAT BIOTECH: PRODUCT LAUNCHES & APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 386 BHARAT BIOTECH: DEALS (JANUARY 2020–MARCH 2024)
13.1.16 NOVAVAX
TABLE 387 NOVAVAX: COMPANY OVERVIEW
FIGURE 56 NOVAVAX: COMPANY SNAPSHOT (2023)
TABLE 388 NOVAVAX: PRODUCTS OFFERED (VACCINES)
TABLE 389 NOVAVAX: PRODUCT APPROVALS (JANUARY 2020–MARCH 2024)
TABLE 390 NOVAVAX: DEALS (JANUARY 2020–MARCH 2024)
13.1.17 INOVIO PHARMACEUTICALS
TABLE 391 INOVIO PHARMACEUTICALS: COMPANY OVERVIEW
FIGURE 57 INOVIO PHARMACEUTICALS: COMPANY SNAPSHOT (2023)
TABLE 392 INOVIO PHARMACEUTICALS: PRODUCTS OFFERED (VACCINES)
TABLE 393 INOVIO PHARMACEUTICALS: DEALS (JANUARY 2020–MARCH 2024)
TABLE 394 INOVIO PHARMACEUTICALS: OTHER DEVELOPMENTS (JANUARY 2020–MARCH 2024)
13.2 OTHER PLAYERS
13.2.1 SINOVAC
13.2.2 INCEPTA PHARMACEUTICALS LTD.
13.2.3 VALNEVA SE
13.2.4 VBI VACCINE INC.
13.2.5 BIO FARMA
13.2.6 MICROGEN
13.2.7 ZHI FEI BIOLOGICAL
13.2.8 INDIAN IMMUNOLOGICALS LIMITED
*Details on Business Overview, Products Offered, Recent Developments, MnM View, Right to win, Strategic choices made, Weaknesses and competitive threats might not be captured in case of unlisted companies.
14 APPENDIX (Page No. - 410)
14.1 DISCUSSION GUIDE
14.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
14.3 CUSTOMIZATION OPTIONS
14.4 RELATED REPORTS
14.5 AUTHOR DETAILS



 Generating Response ...
Generating Response ...







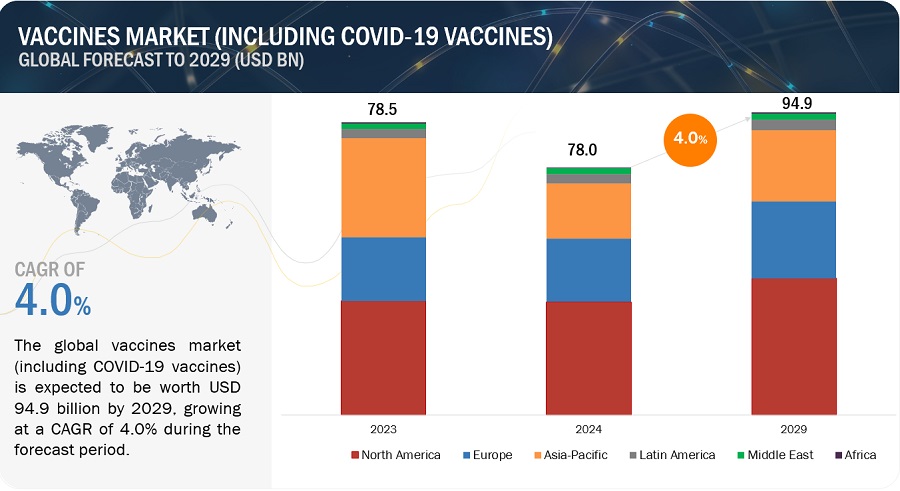
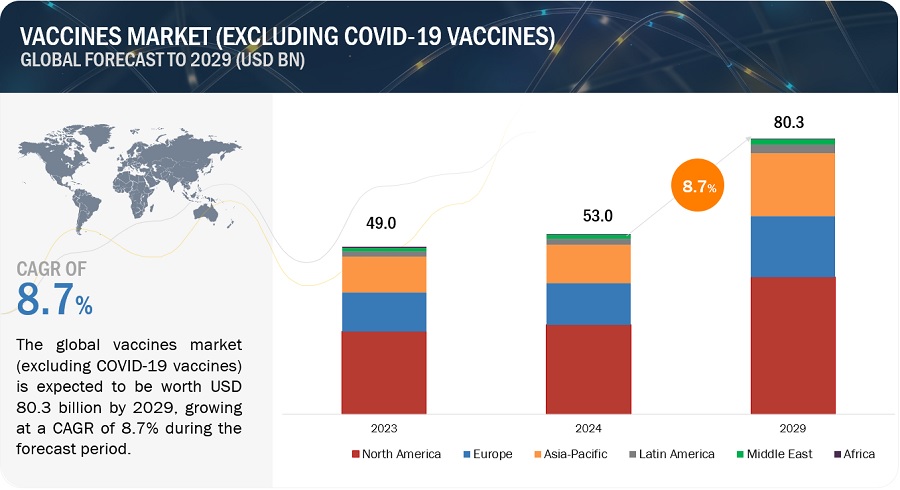
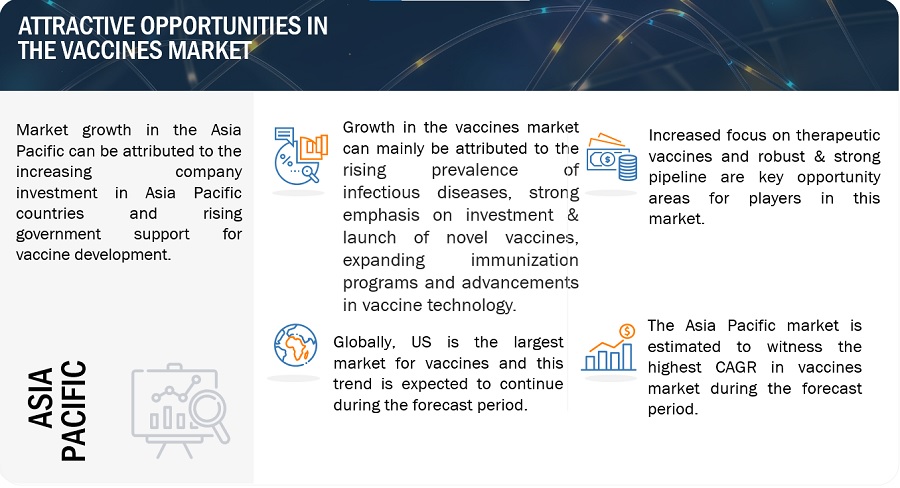
Growth opportunities and latent adjacency in Vaccines Market
What are the growing opportunities in Vaccines Market Size, Share, Growth, Covid-19 Impact Analysis, forecasts to 2028 ?
Which are some of the significant growth strategies adopted by leading companies in this market?
According to this report which are the top 3 companies operating in the global vaccine market?
How the Asia Pacific region in the Global Vaccines Market is Expecting a Highest Growth Rate During the Forecast Period?
Which end user segment holds the largest share of the Global Vaccines Market?