Bio-Implants Market - by Type (Cardiovascular, Spine, Orthopedics, Trauma, Dental), by ROA (Surgical/Injectable), by Origin (Allo/Auto/Xenograft, Synthetic) & Materials (Ceramics, Biomaterial, Alloys, Polymers) - Global Trends &, Forecasts till 2017
Bio-implants can be defined as prostheses used to regularize physiological functions. They are made up of biosynthetic materials like collagen, and tissue-engineered products like artificial skin or tissues. Most bioengineered products like cardiac pacemakers and orthopedic artificial implants are also covered under bio-implants, since they are implanted entirely in the patients body.
Bio-implants are mostly classified at a broader level as:
- Biological implants (cell therapy, bioartificial organs and tissue engineering)
- Biologised implants (technical implants, biohybrid systems, in vivo cell lining)
- Biofunctionalized implants (drug eluting stents, surface functionalized implants)
According to the Centers for Disease Control and Prevention (CDC), there were 40 million people aged 65 and above in the United States in 2010, accounting for 13% of the total population. The older population in 2030 is projected to be twice as large as in 2000, growing from 35 million to 72 million and representing nearly 20% of the total U.S. population. This growth in aging population is expected to push the growth of the dental, reconstructive joint replacement, ophthalmic, and neuromodulation implants markets.
This report describes the growth opportunities in the global bio-implants market. It focuses on the increase in demand for technologically advanced products that may help in faster recovery and better efficacy of physiological body functions.
The global bio-implants market is estimated to be $94.1 billion in 2012 and is poised to grow at a CAGR of 7.3% from 2012 to 2017. Rise in the aging population and increase in incidences of chronic diseases like osteoarthritis, old age-related trauma, cardiovascular diseases, neuropathic disorders (Alzheimers disease and Parkinsons), and congenital disorders and deformities is expected to trigger the growth of this market.
The major players in this market are Stryker Corporation (U.S.), Synthes (U.S.), Boston Scientific Corporation (U.S.), Biomet, Inc. (U.S.), Smith & Nephew (U.K.), Medtronic (U.S.), St. Jude Medical, Inc. (U.S.), Tornier N.V. (The Netherlands), and others.
Scope of the Report
The global bio-implants market is segmented by types, which include major segments such as orthopedic, cardiovascular, dental, ophthalmic, neurostimulation, and others, and by origin (allograft, autograft, xenograft, and synthetic). Each market is comprehensively analyzed at a granular level by geography (North America, Europe, Asia, and Rest of the World) to provide in-depth information on the global scenario.
Global bio-implants market segmentation
- By types
- Cardiovascular Implants
- Pacing Devices
- Cardiac Resynchronization Therapy Devices (CRTs)
- Implantable Cardioverter Defibrillators (ICDs)
- Implantable Cardiac Pacemakers (ICPs)
- Pacing Accessories
- Pacing Leads
- Pacing Batteries
- Stents & Related Implants
- Coronary Stents
- Drug-Eluting Stents
- Bare-Metal Coronary Stents
- Bioabsorbable stents
- Peripheral Stents
- Endovascular Stent-Grafts
- Renal & Related Stents
- Femoral & Related Stents
- Carotid Stents
- Stent-related Implants
- Synthetic Grafts
- Vascular Grafts
- Peripheral Grafts
- Vena Cava Filters
- Coronary Stents
- Structural Cardiac Implants
- Heart Valves & Accessories
- Tissue Heart Valves
- Mechanical Heart Valves
- Heart Valve Repair Devices
- Ventricular-Assist Devices
- Implantable Heart Monitors
- Insertable Loop Recorders (ILRs)
- Implantable Hemodynamic Monitors (IHMs)
- Pacing Devices
- Spinal Implants
- Thoracolumbar Implants
- Intervertebral Spacers
- Machined Allograft Spacers
- Cervical Implants
- Motion Preservation Implants
- Implantable Spinal Stimulators
- Orthopedics & Trauma
- Reconstructive Joint Replacements
- Knee Replacement Implants
- Hip Replacement Implants
- Extremities
- Shoulder Implants
- Elbow Replacements
- Ankle Implants
- Other Joint replacements fusion products
- Orthobiologics
- Hyaluronic Acid
- Bone Substitutes
- Bone Growth Factors
- Bone Cement
- Tissue Implants
- Trauma Implants
- Internal Trauma Fixation Devices
- Craniomaxillofacial Fixation Devices
- Implantable Trauma Stimulators
- Sports Medicine
- Reconstructive Joint Replacements
- Dental
- Plate form dental implants
- Root form dental implants
- Ophthalmic Implants
- Intraocular Lens
- Glaucoma & Other Lenses
- Neurostimulators Implants
- Cortical Stimulators
- Deep Brain Stimulators
- Sacral Nerve Stimulators
- Spinal Cord Stimulators
- Vagus Nerve Stimulators
- Others
- Gynecological Devices
- Drug Implants
- Otolaryngeal Implants
- Cosmetic Implants
- Gastroenterological Implants
- Urological Implants
- Skin & Wound Care
- Cardiovascular Implants
- By origin
- Allograft
- Autograft
- Xenograft
- Synthetic
- By materials
- Ceramics (zirconium)
- Biomaterial metal (titanium, gold, silver, platinum)
- Alloys
- Polymers (natural polymers & synthetic polymers)
- By geography
- North America
- Europe
- Asia
- RoW (Rest of the World)
The use of bio-implants to restore biological structures of the body dates back to the beginning of the 20th century. The early applications of bio-implants included replacement, repair and restoration of hard tissues such as bones, as well as soft tissues including organs, and skin. Over the century, these have evolved and are now used for not just structural revamps, but for various purposes such as for delivering drugs internally to the body and for hormone replacement. They have vast applications and also include electronic battery-operated devices, which help correct the pace of heart beats as well as send interference signals to the nervous system. Their superiority to drugs in treating certain illnesses has been acknowledged globally. Considering the continued quest for better, safer and more effective implants, dealing with diseases is expected to become easier, more cost effective and faster.
The most preferred bio-implants are those of biological origin as they are easily compatible with the body environment. However, synthetic bio-implants are also being increasingly used. Advances in tissue engineering have enabled researchers to prepare artificial prostheses having improved compatibility with the human body The various artificial materials used for preparing bio-implants include metals, ceramics, and special plastic or polymers
The overall bio-implants market is being driven by aging populations, rising incidence of diseases and conditions such as obesity, stress, changing lifestyles, increasing awareness, patient acceptance, product innovation, and increased clinical evidence to prove safety and efficacy of implants.
The global bio-implants market was valued at $94.1 billion for 2012; North America has been, and is expected to be, the largest region for this market, Europe being the second, followed by Asia. The Asian region is set to record the highest CAGR in the global bio-implants market.
The dominant players in the global bio-implants market include Medtronic, St. Jude Medical, Stryker, Boston Scientific, DePuy Synthes, C R Bard, Gore (WL) & Associates Incorporated, Abbott Laboratories, Braun (B.) Melsungen AG, Edwards Lifesciences Corporation Sorin SpA, Zimmer Holdings, Nobel Biocare, Straumann, Dentsply/Astratech, Biomet/3i, Novartis(Alcon), Bausch & Lomb, Cyberonics, Johnson & Johnson, Integra Life Sciences, and Smith & Nephew.
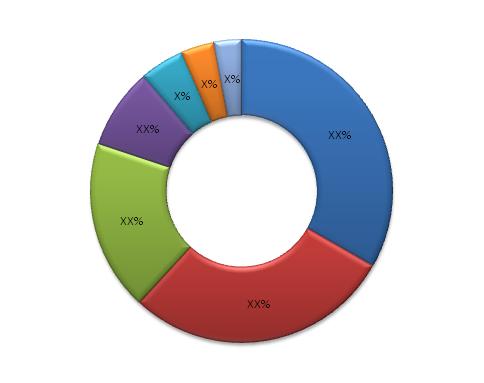
Global Bio-Implants Market, By Types, 2012 - 2017
Source: MarketsandMarkets Analysis

TABLE OF CONTENTS
1 INTRODUCTION
1.1 KEY TAKE-AWAYS
1.2 REPORT DESCRIPTION
1.3 MARKETS COVERED
1.4 STAKEHOLDERS
1.5 RESEARCH METHODOLOGY
1.5.1 MARKET SIZE
1.5.2 MARKET SHARE
1.5.3 KEY DATA POINTS FROM SECONDARY SOURCES
1.5.4 KEY DATA POINTS FROM PRIMARY SOURCES
1.5.5 ASSUMPTIONS
2 EXECUTIVE SUMMARY
3 MARKET OVERVIEW
3.1 INTRODUCTION
3.2 EVOLUTION
3.3 MARKET SEGMENTATION
3.4 MARKET DYNAMICS
3.4.1 DRIVERS
3.4.1.1 Rise in aging population triggers market growth
3.4.1.2 Innovation in implants technologies is driving the growth
3.4.1.3 Changing lifestyles to propel the demand for advanced bio-implants
3.4.1.4 Increased awareness about cosmetic implants
3.4.1.5 Non-surgical bio-implants to propel growth in this market
3.4.1.6 Emerging preference for MIS surgeries
3.4.2 RESTRAINTS
3.4.2.1 High cost of implants to confine the growth of the bio-implants market
3.4.2.2 Hostile reimbursement issues to curb the growth of the bio-implants market
3.5 MARKET SHARE ANALYSIS
3.5.1 CARDIAC
3.5.2 ORTHOPAEDICS
3.5.3 DENTAL
3.5.4 SPINAL
3.5.5 NEUROSTIMULATION
3.5.6 OPHTHALMIC
3.6 BIO-IMPLANTS, BY NON-SURGICAL MODE OF ADMINISTRATION
3.6.1 INJECTABLE FILLERS/BIOMATERIALS
3.6.2 OSTEOGENIC INJECTABLE BONE SUBSTITUTES
3.6.3 VISCOSUPPLEMENTATION
4 BIO-IMPLANTS MARKET, BY TYPES
4.1 INTRODUCTION
4.2 CARDIOVASCULAR IMPLANTS
4.2.1 PACING DEVICES
4.2.1.1 Cardiac Resynchronization Therapy Devices (CRTs)
4.2.1.2 Implantable Cardioverter Defibrillators (ICDs)
4.2.1.3 Implantable Cardiac Pacemakers (ICPs)
4.2.1.4 Pacing accessories
4.2.1.4.1 Pacing leads
4.2.1.4.2 Pacing batteries
4.2.2 STENTS & RELATED IMPLANTS
4.2.2.1 Coronary stents
4.2.2.1.1 Drug-eluting stents
4.2.2.1.2 Bare-metal coronary stents
4.2.2.1.3 Bioabsorbable stents
4.2.2.2 Peripheral stents
4.2.2.2.1 Endovascular stent-grafts
4.2.2.2.2 Renal & related stents
4.2.2.2.3 Femoral & related stents
4.2.2.2.4 Carotid stents
4.2.2.3 Stent-related implants
4.2.2.3.1 Synthetic grafts
4.2.2.3.2 Peripheral vascular grafts
4.2.2.3.3 Vena cava filters
4.2.3 STRUCTURAL CARDIAC IMPLANTS
4.2.3.1 Heart valves & accessories
4.2.3.1.1 Tissue heart valves
4.2.3.1.2 Mechanical heart valves
4.2.3.1.3 Heart valve repair devices
4.2.3.2 Ventricular-assist devices
4.2.3.3 Implantable Heart Monitors/Insertable Loop Recorders (ILRs)
4.3 SPINAL IMPLANTS
4.3.1 THORACOLUMBAR IMPLANTS
4.3.2 INTERVERTEBRAL SPACERS (BONE & NON-BONE)
4.3.3 MACHINED ALLOGRAFT SPACERS
4.3.4 CERVICAL IMPLANTS
4.3.5 MOTION PRESERVATION IMPLANTS
4.3.6 IMPLANTABLE SPINAL STIMULATORS
4.4 ORTHOPEDICS & TRAUMA
4.4.1 RECONSTRUCTIVE JOINT REPLACEMENTS
4.4.1.1 Knee replacement implants
4.4.1.2 Hip replacement implants
4.4.1.3 Extremities
4.4.1.3.1 Elbow replacement implants
4.4.1.3.2 Ankle implants
4.4.1.3.3 Other joint replacement & fusion products
4.4.2 ORTHOBIOLOGICS
4.4.2.1 Hyaluronic acid
4.4.2.2 Bone substitute
4.4.2.3 Bone growth factors
4.4.2.4 Bone cement
4.4.2.5 Tissue implants
4.4.3 TRAUMA IMPLANTS
4.4.3.1 Internal trauma fixation devices
4.4.3.2 Craniomaxillofacial fixation devices
4.4.3.3 Implantable trauma stimulators
4.4.3.4 External fixators
4.4.3.5 Others
4.4.4 SPORTS MEDICINE
4.5 DENTAL
4.5.1 PLATE FORM DENTAL IMPLANTS
4.5.2 ROOT FORM DENTAL IMPLANTS
4.6 OPHTHALMIC IMPLANTS
4.6.1 INTRAOCULAR LENS
4.6.2 GLAUCOMA IMPLANTS
4.7 NEUROSTIMULATORS IMPLANTS
4.7.1 DEEP BRAIN STIMULATORS
4.7.2 SACRAL NERVE STIMULATORS
4.7.3 SPINAL CORD STIMULATORS
4.7.4 VAGUS NERVE STIMULATORS
4.7.5 OTHER NEUROSTIMULATION
4.8 OTHER IMPLANTS
4.8.1 GYNECOLOGICAL DEVICES
4.8.2 DRUG IMPLANTS
4.8.3 OTOLARYNGEAL IMPLANTS
4.8.4 COSMETIC IMPLANTS
4.8.5 GASTROENTEROLOGICAL IMPLANTS
4.8.6 UROLOGICAL IMPLANTS
4.8.7 SKIN & WOUND CARE
5 BIO-IMPLANTS MARKET, BY ORIGIN
5.1 INTRODUCTION
5.2 ALLOGRAFT
5.3 AUTOGRAFT
5.4 XENOGRAFT
5.5 SYNTHETIC
6 BIO-IMPLANTS MARKET, BY MATERIALS
6.1 INTRODUCTION
6.2 CERAMICS
6.3 BIOMATERIAL METALS (TITANIUM, GOLD, SILVER & PLATINUM)
6.4 ALLOYS
6.5 POLYMERS
6.6 BIOLOGICAL MATERIALS
7 GEOGRAPHIC ANALYSIS
7.1 INTRODUCTION
7.2 NORTH AMERICA
7.3 EUROPE
7.4 ASIA
7.5 ROW
8 COMPETITIVE LANDSCAPE
8.1 INTRODUCTION
8.2 NEW PRODUCT LAUNCH IS THE MOST PREFERRED STRATEGY AMONGST PLAYERS
8.3 TOP TWO PLAYERS TOGETHER ACCOUNT FOR ~20% OF THE MARKET DEVELOPMENTS
9 COMPANY PROFILES
9.1 AAP IMPLANTATE AG
9.1.1 OVERVIEW
9.1.2 FINANCIALS
9.1.3 PRODUCTS & SERVICES
9.1.4 STRATEGY
9.1.5 DEVELOPMENTS
9.2 ABBOTT LABORATORIES
9.2.1 OVERVIEW
9.2.2 FINANCIALS
9.2.3 PRODUCTS & SERVICES
9.2.4 STRATEGY
9.2.5 DEVELOPMENTS
9.3 BAUSCH AND LOMB INCORPORATED
9.3.1 OVERVIEW
9.3.2 FINANCIALS
9.3.3 PRODUCTS & SERVICES
9.3.4 STRATEGY
9.3.5 DEVELOPMENTS
9.4 BIOMET, INC.
9.4.1 OVERVIEW
9.4.2 FINANCIALS
9.4.3 PRODUCTS & SERVICES
9.4.4 STRATEGY
9.4.5 DEVELOPMENTS
9.5 BIOTRONIK SE & CO.KG
9.5.1 OVERVIEW
9.5.2 FINANCIALS
9.5.3 PRODUCTS & SERVICES
9.5.4 STRATEGY
9.5.5 DEVELOPMENTS
9.6 BOSTON SCIENTIFIC CORPORATION
9.6.1 OVERVIEW
9.6.2 FINANCIALS
9.6.3 PRODUCTS & SERVICES
9.6.4 STRATEGY
9.6.5 DEVELOPMENTS
9.7 COOK GROUP, INC.
9.7.1 OVERVIEW
9.7.2 FINANCIALS
9.7.3 PRODUCTS & SERVICES
9.7.4 STRATEGY
9.7.5 DEVELOPMENTS
9.8 C.R. BARD, INC.
9.8.1 OVERVIEW
9.8.2 FINANCIALS
9.8.3 PRODUCTS & SERVICES
9.8.4 STRATEGY
9.8.5 DEVELOPMENTS
9.9 EDWARDS LIFESCIENCES CORPORATION
9.9.1 OVERVIEW
9.9.2 FINANCIALS
9.9.3 PRODUCTS & SERVICES
9.9.4 STRATEGY
9.9.5 DEVELOPMENTS
9.10 ENDO HEALTH SOLUTIONS, INC.
9.10.1 OVERVIEW
9.10.2 FINANCIALS
9.10.3 PRODUCTS & SERVICES
9.10.4 STRATEGY
9.10.5 DEVELOPMENTS
9.11 JOHNSON & JOHNSON
9.11.1 OVERVIEW
9.11.2 FINANCIALS
9.11.3 PRODUCTS & SERVICES
9.11.4 STRATEGY
9.11.5 DEVELOPMENTS
9.12 INTEGRA LIFESCIENCES HOLDINGS CORPORATION
9.12.1 OVERVIEW
9.12.2 FINANCIALS
9.12.3 PRODUCTS & SERVICES
9.12.4 STRATEGY
9.12.5 DEVELOPMENTS
9.13 INTEGRATED ORBITAL IMPLANTS
9.13.1 OVERVIEW
9.13.2 FINANCIALS
9.13.3 PRODUCTS & SERVICES
9.13.4 STRATEGY
9.14 LIFENET HEALTH, INC.
9.14.1 OVERVIEW
9.14.2 FINANCIALS
9.14.3 PRODUCTS & SERVICES
9.14.4 STRATEGY
9.14.5 DEVELOPMENTS
9.15 MEDTRONIC, INC.
9.15.1 OVERVIEW
9.15.2 FINANCIALS
9.15.3 PRODUCTS & SERVICES
9.15.4 STRATEGY
9.15.5 DEVELOPMENTS
9.16 MIMEDX GROUP, INC.
9.16.1 OVERVIEW
9.16.2 FINANCIALS
9.16.3 PRODUCTS & SERVICES
9.16.4 STRATEGY
9.16.5 DEVELOPMENTS
9.17 ORTHOFIX INTERNATIONAL N.V.
9.17.1 OVERVIEW
9.17.2 FINANCIALS
9.17.3 PRODUCTS & SERVICES
9.17.4 STRATEGY
9.17.5 DEVELOPMENTS
9.18 SMITH & NEPHEW, PLC
9.18.1 OVERVIEW
9.18.2 FINANCIALS
9.18.3 PRODUCTS & SERVICES
9.18.4 STRATEGY
9.18.5 DEVELOPMENTS
9.19 SORIN S.P.A.
9.19.1 OVERVIEW
9.19.2 FINANCIALS
9.19.3 PRODUCTS & SERVICES
9.19.4 STRATEGY
9.19.5 DEVELOPMENTS
9.20 ST. JUDE MEDICAL, INC.
9.20.1 OVERVIEW
9.20.2 FINANCIALS
9.20.3 PRODUCTS & SERVICES
9.20.4 STRATEGY
9.20.5 DEVELOPMENTS
9.21 STRYKER CORPORATION
9.21.1 OVERVIEW
9.21.2 FINANCIALS
9.21.3 PRODUCTS & SERVICES
9.21.4 STRATEGY
9.21.5 DEVELOPMENTS
9.22 THORATEC CORPORATION
9.22.1 OVERVIEW
9.22.2 FINANCIALS
9.22.3 PRODUCTS & SERVICES
9.22.4 STRATEGY
9.22.5 DEVELOPMENTS
9.23 TORNIER N.V.
9.23.1 OVERVIEW
9.23.2 FINANCIALS
9.23.3 PRODUCTS & SERVICES
9.23.4 STRATEGY
9.23.5 DEVELOPMENTS
9.24 W.L.GORE & ASSOCIATES, INC.
9.24.1 OVERVIEW
9.24.2 FINANCIALS
9.24.3 PRODUCTS & SERVICES
9.24.4 STRATEGY
9.24.5 DEVELOPMENTS
9.25 WRIGHT MEDICAL GROUP INCORPORATED
9.25.1 OVERVIEW
9.25.2 FINANCIALS
9.25.3 PRODUCTS & SERVICES
9.25.4 STRATEGY
9.25.5 DEVELOPMENTS
9.26 ZIMMER HOLDINGS, INC.
9.26.1 OVERVIEW
9.26.2 FINANCIALS
9.26.3 PRODUCTS & SERVICES
9.26.4 STRATEGY
9.26.5 DEVELOPMENTS
LIST OF TABLES
TABLE 1 GLOBAL BIO-IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 2 GLOBAL CARDIOVASCULAR IMPLANTS MARKET, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 3 CARDIOVASCULAR IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 4 GLOBAL PACING DEVICES MARKET, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 5 PACING DEVICES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 6 CARDIAC RESYNCHRONIZATION THERAPY DEVICES MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 7 IMPLANTABLE CARDIOVERTER DEFIBRILLATORS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 8 IMPLANTABLE CARDIAC PACEMAKER MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 9 PACING ACCESSORIES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 10 GLOBAL STENTS AND RELATED IMPLANTS MARKET, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 11 STENTS AND RELATED IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 12 GLOBAL CORONARY STENTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 13 CORONARY STENTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 14 DRUG-ELUTING STENTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 15 BARE-METAL STENTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 16 BIOABSORBABLE STENTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 17 GLOBAL PERIPHERAL STENTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 18 PERIPHERAL STENTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 19 ENDOVASCULAR STENT-GRAFTS (ILEAC STENTS) MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 20 RENAL & RELATED STENTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 21 FEMORAL & RELATED STENTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 22 CAROTID STENTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 23 GLOBAL STENT RELATED IMPLANTS MARKET, BY PRODUCTS, 2010 2017 ($MILLION)
TABLE 24 SYNTHETIC GRAFTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 25 PERIPHERAL VASCULAR GRAFTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 26 VENA CAVA FILTERS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 27 GLOBAL STRUCTURAL CARDIAC IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 28 STRUCTURAL CARDIAC IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 29 GLOBAL HEART VALVES AND ACCESSORIES MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 30 HEART VALVE AND ACCESSORIES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 31 TISSUE HEART VALVES MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 32 MECHANICAL HEART VALVES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 33 HEART VALVE REPAIR DEVICES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 34 VENTRICULAR ASSIST DEVICES MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 35 IMPLANTABLE HEART MONITORS/INSERTABLE LOOP RECORDERS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 36 GLOBAL SPINAL IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 37 SPINAL IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 38 THORACOLUMBAR IMPLANT MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 39 INTERVERTEBRAL SPACERS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 40 MACHINED BONE/MACHINED ALLOGRAFT SPACERS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 41 CERVICAL IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 42 MOTION PRESERVATION IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 43 SPINAL STIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 44 GLOBAL ORTHOPAEDICS & TRAUMA IMPLANTS, BY TYPES, 2010 - 1017 ($MILLION)
TABLE 45 ORTHOPAEDICS & TRAUMA IMPLANTS, BY GEOGRAPHY, 2010 - 1017 ($MILLION)
TABLE 46 GLOBAL RECONSTRUCTIVE JOINT REPLACEMENT IMPLANTS MARKET, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 47 RECONSTRUCTIVE JOINT REPLACEMENT IMPLANTS MARKET, BY GEOGRAPHY, 2010 -2017 ($MILLION)
TABLE 48 KNEE REPLACEMENT IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 49 HIP REPLACEMENT IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 50 GLOBAL EXTREMITIES IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 51 EXTREMITIES IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 52 ELBOW REPLACEMENT IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 53 ANKLE REPLACEMENT AND FUSION PRODUCTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 54 OTHER JOINT REPLACEMENT AND FUSION PRODUCTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 55 GLOBAL ORTHOBIOLOGICS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 56 ORTHOBIOLOGICS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 57 HYALURONIC ACID MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 58 BONE SUBSTITUTES MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 59 BONE GROWTH FACTORS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 60 BONE CEMENT MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 61 TISSUE IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 62 GLOBAL TRAUMA IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 63 TRAUMA IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 64 INTERNAL TRAUMA FIXATION DEVICES MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 65 CRANIOMAXILLOFACIAL FIXATION DEVICES MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 66 IMPLANTABLE TRAUMA STIMULATORS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 67 EXTERNAL FIXATORS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 68 OTHER EXTERNAL FIXATORS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 69 SPORTS MEDICINE IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 70 GLOBAL DENTAL IMPLANTS MARKET, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 71 DENTAL IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 72 PLATE FORM DENTAL IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 73 ROOT FORM DENTAL IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 74 GLOBAL OPHTHALMIC IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 75 OPHTHALMIC IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 76 INTRAOCULAR LENS IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 77 GLAUCOMA IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 78 GLOBAL NEUROSTIMULATORS IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 79 NEUROSTIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 80 DEEP BRAIN STIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 81 SACRAL NERVE STIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 82 SPINAL CORD STIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 - 2017 ($MILLION)
TABLE 83 VAGUS NERVE STIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 84 OTHER NEUROSTIMULATORS IMPLANTS MARKET, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 85 GLOBAL OTHER IMPLANTS MARKET, BY TYPES, 2010 2017 ($MILLION)
TABLE 86 GLOBAL BIO-IMPLANTS MARKET REVENUE, BY ORIGIN, 2010 2017 ($MILLION)
TABLE 87 GLOBAL BIO-IMPLANTS MARKET REVENUE, BY MATERIALS, 2010 2017 ($MILLION)
TABLE 88 BIO-IMPLANTS MARKET REVENUE, BY GEOGRAPHY, 2010 2017 ($MILLION)
TABLE 89 NORTH AMERICA: BIO-IMPLANTS MARKET REVENUE, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 90 EUROPE: BIO-IMPLANTS MARKET REVENUE, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 91 ASIA: BIO-IMPLANTS MARKET REVENUE, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 92 ROW: BIO-IMPLANTS MARKET REVENUE, BY TYPES, 2010 - 2017 ($MILLION)
TABLE 93 NEW PRODUCT LAUNCH, 2009 - 2012
TABLE 94 AGREEMENTS & COLLABORATIONS, 2009 - 2012
TABLE 95 MERGERS & ACQUISITIONS, 2009 - 2012
TABLE 96 PRODUCTS PIPELINE, 2009 - 2012
TABLE 97 EXPANSION, 2009 - 2012
TABLE 98 APPROVALS, 2009 - 2012
TABLE 99 OTHER DEVELOPMENTS, 2009 - 2012
TABLE 100 AAP IMPLANTATE AG: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 101 AAP IMPLANTATE AG: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 102 AAP IMPLANTATE AG: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 103 ABBOTT LABORATORIES: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 104 ABBOTT LABORATORIES: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 105 ABBOTT LABORATORIES: TOTAL REVENUE, BY GEOGRAPHY, 2009 2011 ($MILLION)
TABLE 106 BIOMET, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 107 BIOMET, INC.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 108 BIOMET, INC.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 109 BOSTON SCIENTIFIC CORPORATION: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 110 BOSTON SCIENTIFIC CORPORATION: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 111 BOSTON SCIENTIFIC CORPORATION: TOTAL REVENUE OF CORONARY STENT SYSTEM, 2009 - 2011 ($MILLION)
TABLE 112 BOSTON SCIENTIFIC CORPORATION: TOTAL REVENUE OF CRM SEGMENT, 2009 - 2011 ($MILLION)
TABLE 113 BOSTON SCIENTIFIC CORPORATION: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 114 C.R. BARD, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 115 C.R. BARD, INC.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 116 C.R. BARD, INC.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 117 EDWARDS LIFESCIENCES CORPORATION: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 118 EDWARDS LIFESCIENCES CORPORATION: TOTAL REVENUE, BY PRODUCTS, 2009 - 2011 ($MILLION)
TABLE 119 EDWARDS LIFESCIENCES CORPORATION: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 120 ENDO HEALTH SOLUTIONS, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 121 ENDO HEALTH SOLUTIONS, INC.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 122 JOHNSON & JOHNSON: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 123 JOHNSON & JOHNSON: TOTAL REVENUE, BY SEGMENTS, 2009 2011 ($MILLION)
TABLE 124 JOHNSON & JOHNSON: MEDICAL DEVICES AND DIAGNOSTICS REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 125 JOHNSON & JOHNSON: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 126 INTEGRA LIFESCIENCES HOLDINGS CORPORATION: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 127 INTEGRA LIFESCIENCES HOLDINGS CORPORATION: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 128 INTEGRA LIFESCIENCES HOLDINGS CORPORATION: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 129 MEDTRONIC, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2010 - 2012 ($MILLION)
TABLE 130 MEDTRONIC, INC.: TOTAL REVENUE, BY SEGMENTS, 2010 - 2012 ($MILLION)
TABLE 131 MEDTRONIC, INC.: TOTAL REVENUE, BY GEOGRAPHY, 2010 - 2012 ($MILLION)
TABLE 132 MIMEDX GROUP, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 133 ORTHOFIX INTERNATIONAL N V: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 134 ORTHOFIX INTERNATIONAL N.V.: TOTAL REVENUE, BY SEGMENTS, 2009 2011 ($MILLION)
TABLE 135 ORTHOFIX INTERNATIONAL N.V.: TOTAL REVENUE OF SPINE SEGMENT, 2009 - 2011 ($MILLION)
TABLE 136 SMITH & NEPHEW, PLC: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 137 SMITH & NEPHEW PLC: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 138 SMITH & NEPHEW, PLC: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 139 SORIN S.P.A.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 140 SORIN S.P.A.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 141 SORIN S.P.A.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 142 ST. JUDE MEDICAL, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2010 ($MILLION)
TABLE 143 ST. JUDE MEDICAL, INC: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 144 ST. JUDE MEDICAL, INC.: TOTAL REVENUE OF CARDIAC RHYTHM MANAGEMENT SEGMENT ($MILLION)
TABLE 145 ST. JUDE MEDICAL, INC.: TOTAL REVENUE OF CARDIOVASCULAR SEGMENT, 2009 - 2011 ($MILLION)
TABLE 146 ST. JUDE MEDICAL, INC.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 147 STRYKER CORPORATION: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 148 STRYKER CORPORATION: TOTAL REVENUE, BY SEGMENTS, 2009-2011 ($MILLION)
TABLE 149 STRYKER CORPORATION: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 150 THORATEC CORPORATION: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 151 THORATEC CORPORATION: TOTAL REVENUE, BY PRODUCT LINE, 2009 - 2011 ($MILLION)
TABLE 152 THORATEC CORPORATION: TOTAL REVENUE, BY GEOGRAPHY, 2009 2011 ($MILLION)
TABLE 153 TORNIER N.V.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 154 TORNIER N.V.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 155 TORNIER N.V.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
TABLE 156 WRIGHT MEDICAL GROUP INCORPORATED: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 157 WRIGHT MEDICAL GROUP INCORPORATED: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 158 WRIGHT MEDICAL GROUP INCORPORATED: TOTAL REVENUE, BY GEOGRAPHY, 2009 2011 ($MILLION)
TABLE 159 ZIMMER HOLDINGS, INC.: TOTAL REVENUE AND R&D EXPENDITURE, 2009 - 2011 ($MILLION)
TABLE 160 ZIMMER HOLDINGS, INC.: TOTAL REVENUE, BY SEGMENTS, 2009 - 2011 ($MILLION)
TABLE 161 ZIMMER HOLDINGS, INC.: TOTAL REVENUE, BY GEOGRAPHY, 2009 - 2011 ($MILLION)
LIST OF FIGURES
FIGURE 1 BIO-IMPLANTS MARKET SEGMENTATION
FIGURE 2 MARKET DYNAMICS
FIGURE 3 GLOBAL CARDIAC MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 4 GLOBAL ORTHOPAEDICS MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 5 GLOBAL DENTAL MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 6 GLOBAL SPINAL MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 7 GLOBAL NEUROSTIMULATION MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 8 GLOBAL OPHTHALMIC MARKET SHARE ANALYSIS, BY PLAYERS, 2011
FIGURE 9 KEY GROWTH STRATEGIES, JANUARY 2009 JULY 2012
FIGURE 10 NEW PRODUCT LAUNCH STRATEGY, BY COMPANY, JANUARY 2009 JULY 2012
FIGURE 11 KEY GROWTH STRATEGIES, BY COMPANY, JANUARY 2009 JULY 2012



 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Bio-Implants Market