
Conjugate Vaccine Market Size, Growth, Share & Trends Analysis
Conjugate Vaccines Market by Disease (Pneumococcal, DTP, Meningococcal Disease), Type (Monovalent, Multivalent), End User (Adult, Pediatric) - Forecast to 2030




OVERVIEW
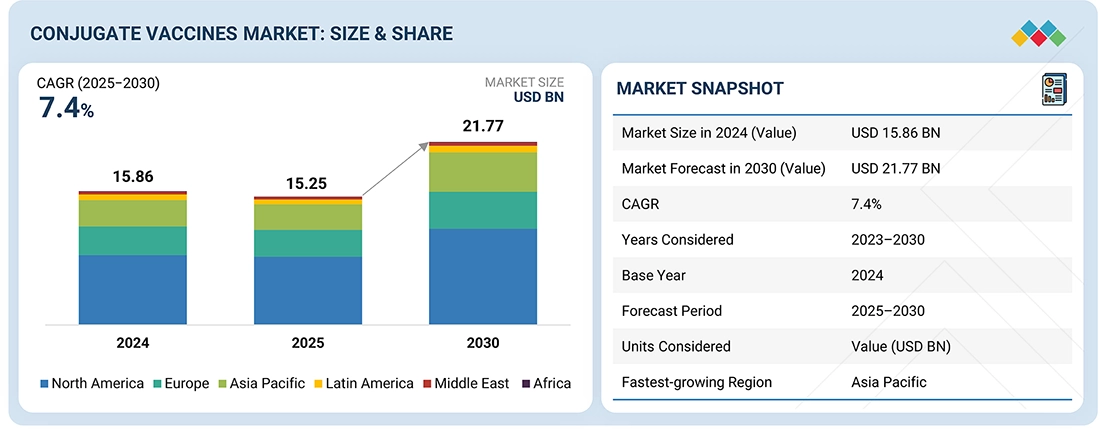
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The conjugate vaccines market is projected to reach USD 21.77 billion by 2030 from USD 15.25 billion in 2025, at a CAGR of 7.4% from 2025 to 2030. The conjugate vaccines market is growing rapidly, driven by the rising prevalence of bacterial infections like pneumococcal, meningococcal, and Hemophilus influenzae type b (Hib), rising government immunization programs and policies, innovations in vaccine technology such as multivalent formulations, increased regulatory approvals for new products, and growing investments in research, development, and international collaborations.
KEY TAKEAWAYS
-
BY COUNTRYThe Asia Pacific is projected to register the highest CAGR of 9.1% in the conjugate vaccines market during the forecast period.
-
BY DISEASEThe pneumococcal disease segment is projected to register the highest CAGR of 8.0% CAGR during the forecast period.
-
BY TYPEThe multivalent vaccines segment held the largest market share of 70% in 2024.
-
BY End UserPediatric vaccines dominated the conjugate vaccines market in 2024.
-
COMPETITIVE LANDSCAPEMerck & Co. (US), Pfizer (US), and GSK (UK) were identified as star players in the conjugate vaccines market, given their strong market share and extensive product portfolio.
-
COMPETITIVE LANDSCAPESinovac (China) and BioFarma (Indonesia), among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The conjugate vaccines market is growing due to a number of key factors, including the strengthening of the healthcare infrastructure, proactive agencies in terms of vaccination programs like the CDC and the FDA, and high awareness about public health. Rising investments in research by leading pharmaceutical players and regulatory approvals for novel products have boosted the adoption of vaccines in preventive health. Advanced conjugate platforms are crucial for combating infectious diseases, enabling effective immunization strategies against bacterial pathogens, such as pneumococcus and meningococcus.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The conjugate vaccines market is undergoing transformation from rapidly evolving scientific, regulatory, and clinical developments, intensifying adoption for targeted immunization against infectious diseases. Novel pathogen vaccines paired with robust trial activity and public health efforts fuel demand for scalable, dependable platforms across hospitals and research institutions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Strong Disease Burden in Target Populations

-
Inclusion in National Immunization Programs
Level
-
Supply Chain and Production Constraints
Level
-
Next-generation Conjugate Products
Level
-
Pricing Pressure and Tender Competition
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Strong Disease Burden in Target Populations
The high prevalence of bacterial infectious diseases, such as pneumococcal disease, meningococcal disease, and Hemophilus influenzae type b (Hib), particularly among infants, young children, the elderly population, and immunocompromised individuals, is a primary driver of the conjugate vaccines market. Rising awareness of vaccine-preventable diseases, coupled with national immunization programs and recommendations from global health organizations, continues to support sustained demand for conjugate vaccines across both developed and emerging markets.
Restraint: Supply Chain and Production Constraints
Conjugate vaccines involve complex, multistep manufacturing processes, including polysaccharide extraction, protein conjugation, and stringent quality control, which limit their scalability for production. Dependence on specialized raw materials, cold-chain logistics, and a limited number of qualified manufacturing facilities can lead to supply bottlenecks. These constraints may lead to delayed deliveries, increased production costs, and difficulties in meeting global demand, particularly in low- and middle-income countries.
Opportunity: Next-generation Conjugate Products
Advancements in conjugation technologies and carrier proteins are creating opportunities for next-generation conjugate vaccines with broader serotype coverage, improved immunogenicity, and longer duration of protection. The development of multivalent and combination conjugate vaccines, along with the expansion into adult and adolescent immunization programs, presents significant growth potential. Additionally, increasing investments in R&D and public–private partnerships are accelerating innovation in this segment.
Challenge: Pricing Pressure and Tender Competition
The conjugate vaccines market faces significant pricing pressure due to government procurement mechanisms, large-scale public tenders, and the growing presence of low-cost manufacturers, particularly in emerging markets. Intense competition for national immunization contracts frequently results in margin compression for manufacturers. Balancing affordability with high production costs and sustained R&D investment remains a key challenge for market participants.
CONJUGATE VACCINE MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Large-scale, cost-efficient manufacturing of conjugate vaccines (pneumococcal, meningococcal, Hib) with WHO-prequalified facilities, enabling high-volume supply to Gavi-, UNICEF-, and government-led immunization programs across emerging markets. | Expands global vaccine access, improves affordability, ensures supply security for national immunization programs, and supports rapid scale-up in outbreak or catch-up vaccination scenarios. |
 |
Advanced conjugate vaccine development and commercialization leveraging proprietary carrier proteins, optimized conjugation chemistry, and strong regulatory and market access capabilities across pediatric and adolescent immunization programs globally. | Delivers differentiated immunogenicity and durability, supports broad serotype coverage, accelerates regulatory approvals, and strengthens long-term disease prevention outcomes in routine immunization schedules. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The conjugate vaccines market is characterized by closely networked collaborations among instrument providers, bioinformatics firms, contract research organizations (CROs), biopharmaceutical developers, and clinic-based facilities. These entities deliver high-throughput manufacturing platforms alongside analytical tools for conjugate vaccines, while research-driven trials occur in CROs and core facilities, aiding hospitals and biopharma firms in assessing vaccine efficacy.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
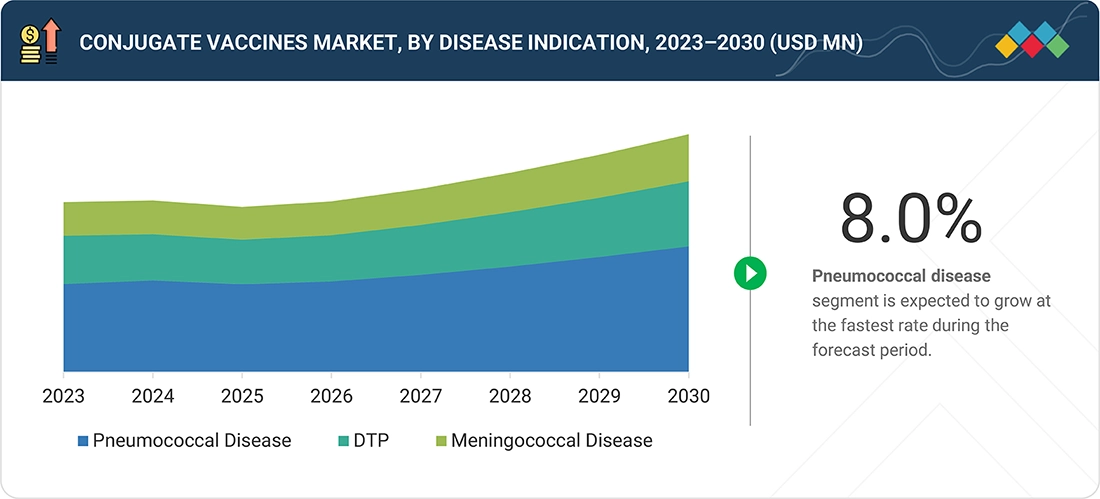
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Conjugate Vaccines Market, by Disease Indication
In 2024, the pneumococcal segment held the largest share of the conjugate vaccines market, driven by the rising incidence of pneumococcal infections, increased government funding for vaccination programs, and heightened public awareness of the serious health risks associated with related complications.
Conjugate Vaccines Market, by Type
In 2024, the multivalent vaccines segment held the largest share of the conjugate vaccines market, primarily due to simplified immunization programs that provide broad protection against multiple serotypes in a single formulation.
Conjugate Vaccines Market, by End User
In 2024, the pediatric end-user segment held the largest share of the conjugate vaccines market due to children's high vulnerability to bacterial infections like pneumococcal, meningococcal, and Hib diseases, widespread inclusion in routine immunization schedules, and strong government-backed vaccination programs worldwide.
REGION
Asia Pacific to be fastest-growing region in market during forecast period
During the forecast period, the Asia Pacific region emerged as one of the fastest-growing market for conjugate vaccines globally, primarily due to the ongoing expansion of commercial production capabilities. The presence of robust pipelines for multivalent pneumococcal and meningococcal conjugates, along with next-generation formulations targeting emerging serotypes, has been further enhanced by significant investments in large CDMO facilities and upgrades by major pharmaceutical companies, such as Serum Institute and Panacea Biotec. This has accelerated the adoption of high-performance manufacturing processes across both new and existing plants.

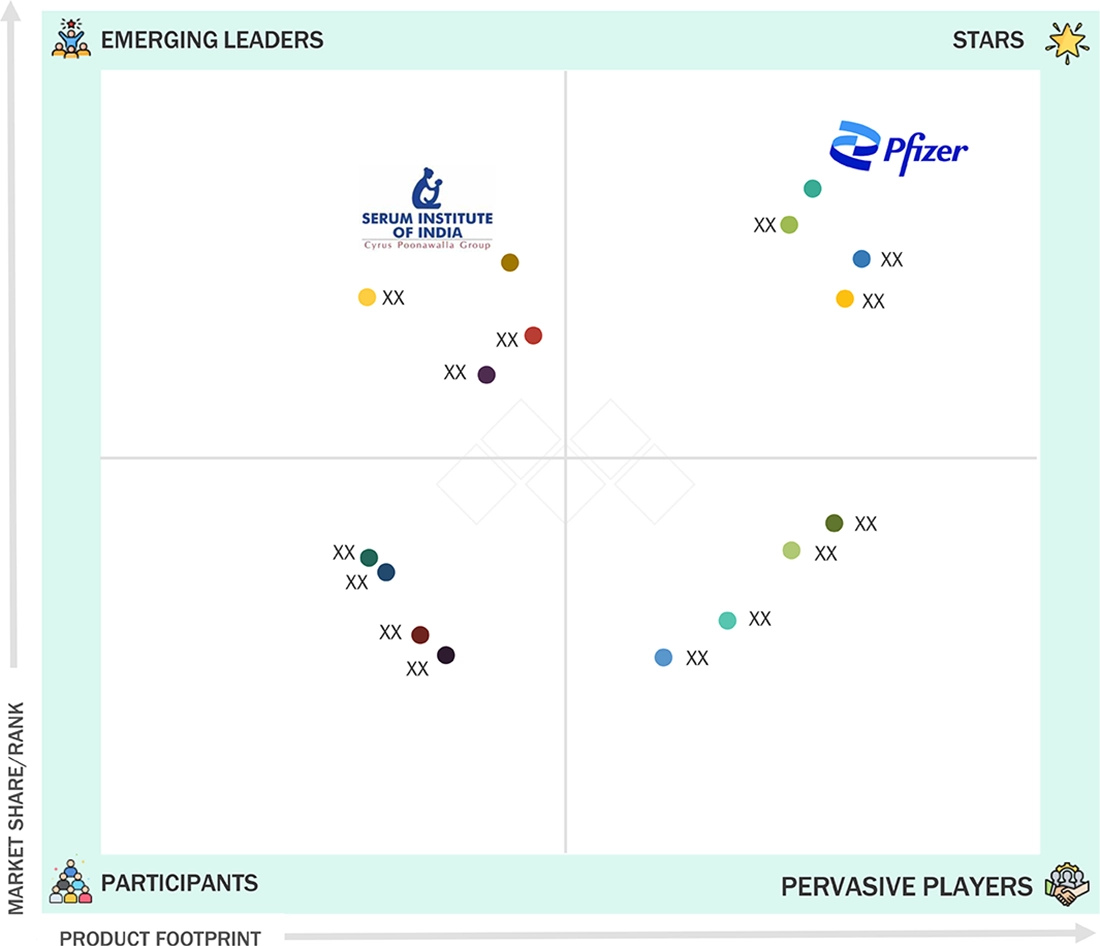
CONJUGATE VACCINE MARKET: COMPANY EVALUATION MATRIX
Pfizer is a Star player in the conjugate vaccines market, significantly influencing industry dynamics with its established multivalent pneumococcal conjugate portfolio. The company has strong penetration in immunization programs across both pediatric and adult segments. In contrast, the Serum Institute of India is an emerging leader making rapid strides as a high-volume, cost-competitive supplier of conjugate vaccines. By leveraging WHO prequalification and forming strategic public-sector partnerships, the Serum Institute is increasingly gaining market share and improving access to pneumococcal and other conjugate formulations in emerging regions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Pfizer Inc. (US)
- Merck & Co., Inc. (US)
- GSK (UK)
- Sanofi (France)
- Serum Institute of India (India)
- Daiichi Sankyo Company Limited (Japan)
- Panacea Biotec (India)
- Biological E Limited (India)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 15.86 Billion |
| Market Forecast in 2030 (Value) | USD 21.77 Billion |
| Growth Rate | CAGR of 7.4 % from 2025-2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | By Disease Indication: Pneumococcal Disease, DTP, Meningococcal Disease, Other Disease Indications I By Type: Monovalent and Multivalent Vaccines I By End User: Pediatric Vaccines and Adult Vaccines |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East, Africa |
| Parent & Related Segment Reports |
Vaccines Market Europe Vaccines Market Respiratory Syncytial Virus (RSV) Vaccines Market Asia Pacific Vaccines Market North America Vaccines Market |
WHAT IS IN IT FOR YOU: CONJUGATE VACCINE MARKET REPORT CONTENT GUIDE
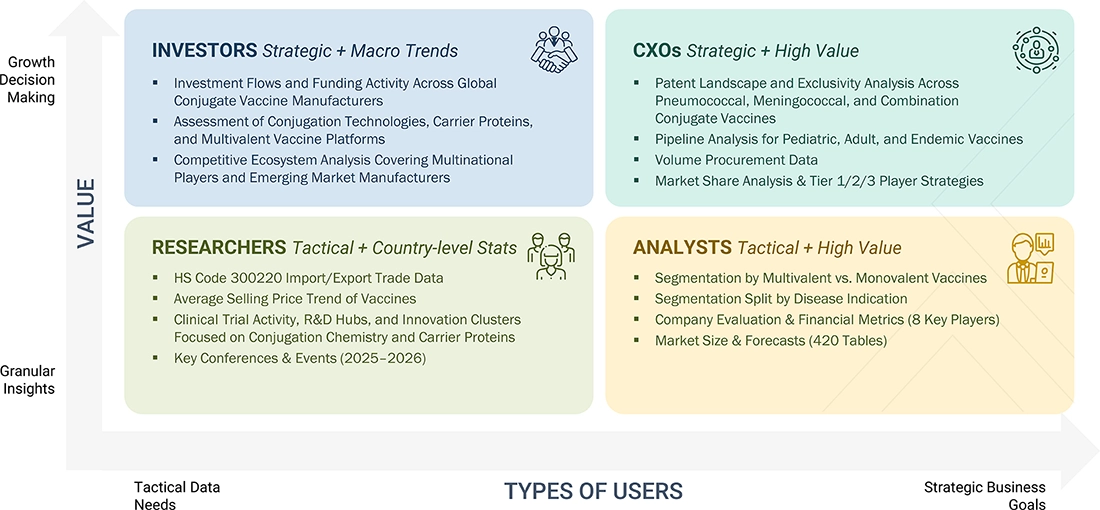
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Regulatory Intelligence & Compliance | Comprehensive gap analyses against BLA/MAA modules specific to conjugate vaccines, predictive modeling of CMC review timelines, and bridging strategies for post-approval serotype expansions under SUPAC guidelines | Enables rapid pipeline expansion, de-risked development, and access to advanced technologies while improving commercialization success in the US and Canadian markets |
| Clinical Development |
|
Boosts trial success through robust evidence, secures preferential regulatory status, and expedites label expansions efficiently |
RECENT DEVELOPMENTS
- January 2026 : Merck completed the acquisition of Cidara Therapeutics to bolster its conjugate vaccine pipeline, enhancing capabilities against resistant pneumococcal strains.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global vaccines market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive technical, market-oriented, and commercial study of the vaccines market. The secondary sources used for this study include World Health Organization (WHO), the Organization for Economic Co-operation and Development (OECD), National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), the Global Cancer Observatory (GLOBOCAN), the National Institutes of Health (NIH), Center of Disease Control & Prevention (CDC), US Department of Health and Human Services, National Institutes of Health (NIH), National Library of Medicine, National Center for Biotechnology Information (NCBI), National Institute of Allergy and Infectious Diseases (NIAID), World Cancer Research Fund International (WCRF International), European Medicines Agency (EMA), The National Medical Products Administration (NMPA), Global Alliance for Conjugate vaccines and Immunization (GAVI), United States Food & Drug Administration (US FDA), Orange book, Purple book, Clinical trials.gov, Pan American Health Organization (PAHO), United Nation International Children’s Emergency Fund (UNICEF), Department of health and Human Services (HHS), and International Society for Conjugate vaccines (ISV). Corporate filings include annual reports, SEC filings, investor presentations, and financial statements; research journals; press releases; and trade, business, and professional associations. Secondary data was collected and analyzed to arrive at the overall size of the global vaccines market, which was validated through primary research. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, among other experts, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects of the market. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were employed to estimate and validate the overall size of the vaccines market. These methods were also widely used to determine the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:

Data Triangulation
After estimating the overall market size through the market size estimation process, the total market was divided into several segments and subsegments. Data triangulation and market breakdown techniques were used where applicable to finalize the overall market analysis and obtain precise statistics for all segments and subsegments. The data was triangulated by examining various factors and trends from both demand and supply sides.
Market Definition
A vaccine is a biologically formulated product designed to trigger active acquired immunity against a specific infectious or malignant disease. Conjugate vaccines work by stimulating the immune system to identify and fight harmful agents, such as viruses or bacteria. They generally consist of parts that resemble the disease-causing microorganism, often in weakened or deactivated forms, along with their toxins or surface proteins. The report solely focuses on human vaccines and does not include veterinary vaccines, which are outside the scope of this study.
Stakeholders
- Vaccine product manufacturers and suppliers
- Distributors and suppliers of vaccine products
- Vaccine research institutes
- Biotechnology and biopharmaceutical companies
- Contract manufacturing organizations (CMOs)
- Contract development and manufacturing organizations (CDMO)
- Suppliers and distributors of pharmaceutical products
- Research and development (R&D) companies
- Drug Manufacturers, Vendors, and Distributors
- Immunization centres
- Hospitals and laboratories
- Trade associations and industry bodies
- Regulatory bodies and government organizations
- Venture capitalists and investors
- Hospitals
- Specialty Clinics
Report Objectives
- To define, describe, and forecast the vaccines market by technology, type, disease indication, route of administration, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall vaccines market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments, such as product launches, agreements, partnerships, acquisitions, regulatory approvals, and research & development activities
- To analyze and provide funding & investment activities, brand/product comparative analysis, and vendor valuation & financial metrics of the vaccines market.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Conjugate Vaccines Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Conjugate Vaccines Market