The study involved major activities in estimating the current market size of the closed system transfer devices market. Exhaustive secondary research was done to collect information on the closed system transfer devices market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, such as top-down and bottom-up, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were used to estimate the market size of the segments and subsegments of the closed system transfer devices market.
Secondary Research
This research study involved the wide use of secondary sources, directories, and databases such as Dun & Bradstreet, Bloomberg Businessweek, Factiva, whitepapers, and companies’ house documents. Secondary research was undertaken to identify and collect information for this extensive, technical, market-oriented, and commercial study of the closed system transfer devices market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various supply- and demand-side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, engineers, and related key executives from various companies and organizations operating in the closed system transfer devices market. Primary sources from the demand side included hospitals, clinics, researchers, lab technicians, purchase managers, and stakeholders in corporate & government bodies.
Breakdown of Primary Interviews
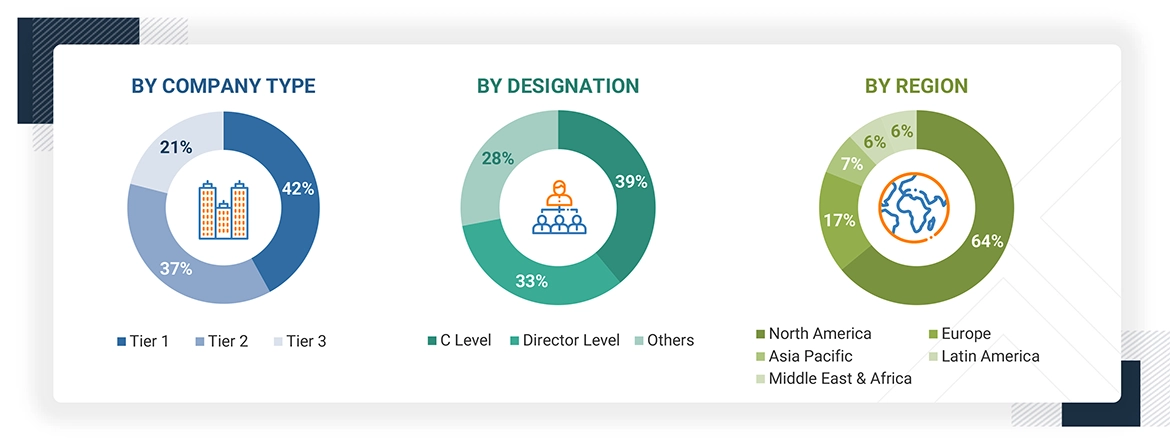
Note 1: C-level primaries include CEOs, COOs, and CTOs.
Note 2: Others include sales managers, marketing managers, and product managers.
Note 3: Companies are classified into tiers based on their total revenue. As of 2023: Tier 1=>USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, Tier 3=< USD 500 million
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the closed system transfer devices market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:

Data Triangulation
The entire market was split into five segments when the market size was determined. Data triangulation and market breakdown processes were used where necessary to complete the entire market engineering process and arrive at precise statistics for all segments.
Approach to derive the market size and estimate market growth.
Using secondary data from paid and unpaid sources, the market rankings for the major players were determined following a thorough analysis of their sales of CSTD. Due to data restrictions, the revenue share in certain cases was determined after a thorough analysis of the product portfolio of big corporations and their individual sales performance. This information was verified at each stage by in-depth interviews with professionals in the field.
Market Definition
Closed system transfer devices are highly specialized devices that mechanically prohibit the transfer of environmental contaminants into a system and the escape of hazardous drug or vapor concentrations outside the system. These devices are uniquely designed to protect healthcare providers and patients from exposure to hazardous drugs.
Stakeholders
-
CSTD Manufacturers
-
CSTD Suppliers & Distributors
-
Original Equipment Manufacturers (OEMs)
-
Hospitals and Clinics
-
Oncology Centers
-
Medical Device Research and Consulting Firms
-
Contract Research Organizations (CROs)
-
Academic & Research Institutes
-
Government Associations
-
Market Research and Consulting Firms
-
Venture Capitalists and Investors
Report Objectives
-
To define, describe, and forecast the closed system transfer devices market based on closing mechanism, type, component, technology, end user, and region
-
To provide detailed information regarding the major factors influencing the market growth (drivers, restraints, opportunities, and challenges)
-
To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall closed system transfer devices market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
-
To forecast the size of the closed system transfer devices market in five regions, namely, North America (US and Canada), Europe (Germany, UK, France, Spain, Italy, and Rest of Europe), the Asia Pacific (Japan, China, India, Australia & New Zealand, South Korea, and Rest of Asia Pacific), Latin America (Brazil, Mexico, Colombia, and Rest of Latin America), and the Middle East & Africa (GCC Countries, and Rest of Middle East & Africa)
-
To analyze the impact of the economic recession on the growth of the closed system transfer devices market
-
To strategically profile the key players in the global market and comprehensively analyze their core competencies
-
To track and analyze competitive developments, such as product approvals, launches, expansions, and acquisitions, of the leading players in the market



Dr.
May, 2022
Working on standardization in the field of Closed System Transfer Devices Market Segment Growth, Size, Share [2030].
Samantha
Mar, 2022
What is the share of the top companies in the Global Closed System Transfer Devices Market?.
Katherine
Mar, 2022
Which factors are playing vital role in growth of the Global Closed System Transfer Devices Market in different geographies?.