Influenza Diagnostics Market by Product (Test Kits, Instruments), Test Type (Traditional (RIDT, Viral Culture, Serological), Molecular (PCR, INAAT- NASBAT, TMABAS)), End User (Diagnostic Laboratories, Hospitals, Clinics) & Region - Global Forecast to 2026
Market Growth Outlook Summary
The global influenza diagnostics market growth forecasted to transform from $0.8 billion in 2021 to $1.1 billion by 2026, driven by a CAGR of 7.7%. Key factors driving this growth include increasing demand for rapid disease diagnosis, rising influenza prevalence, and advancements in diagnostic technologies. The market is segmented by product (test kits, reagents, instruments), test type (molecular, traditional tests), and end users (diagnostic labs, hospitals). North America holds the largest market share, driven by robust research activities. Major players include Thermo Fisher Scientific, Becton, Dickinson, and Abbott Laboratories. Key developments include FDA approvals for new influenza diagnostic technologies.
Influenza Diagnostics Market Trends
To know about the assumptions considered for the study, Request for Free Sample Report
Influenza Diagnostics Market Dynamics
Driver: Growth in influenza research for diagnostic technologies
The rising prevalence of influenza across the globe has increased R&D efforts towards its effective detection and diagnosis. Most research activity focuses on developing faster and more accurate diagnostic solutions for influenza viruses, leading to market growth. The National Institute of Allergy and Infectious Diseases (NIAID), a part of the US NIH, initiated the Collaborative Influenza Vaccine Innovation Centers (CIVICs) programme to support a broad portfolio of influenza research activities. CIVICs will receive USD 51 million in funding from the NIAID.
Opportunity: Advancements in genomic and proteomic technologies
The Human Genome Project and advances in molecular and biomedical technologies have generated a vast amount of data, which has resulted in the development of a multitude of assays and technologies useful for the diagnosis and management of influenza infections. These new technologies, based on genomic techniques (such as PCR-based) and proteomics (such as microarray-based detection), help discover new influenza viruses. They also enable better surveillance and rapid diagnosis of infectious diseases, which serves as an opportunity for the market.
Restraint: Variabilities in test sensitivity and specificity
Sensitivity and specificity are the major factors that impact the results of influenza diagnostic tests. The antigenic variation of influenza viruses is the main reason behind the complexity of these tests. As a result, there are variabilities in the sensitivity and specificity of influenza diagnostic tests, which can impact the final test results. Due to this factor, false-negative results are more common than false-positive results, especially during peak influenza season, which is a major factor restraining the growth of the influenza diagnostics market. Recently, the FDA cleared rapid influenza diagnostic tests (RIDTs).
Challenge: Stringent regulatory framework
A major challenge that most diagnostic companies face in commercialising their tests is getting Medicare and private health insurers to pay for them. This is important not only to help the decision-making process of physicians within the practise of evidence-based medicine but also to achieve regulatory approvals and reimbursements for the tests. Regulatory approvals for influenza tests have become more stringent in recent years to ensure their efficiency in detecting all types of influenza viruses.
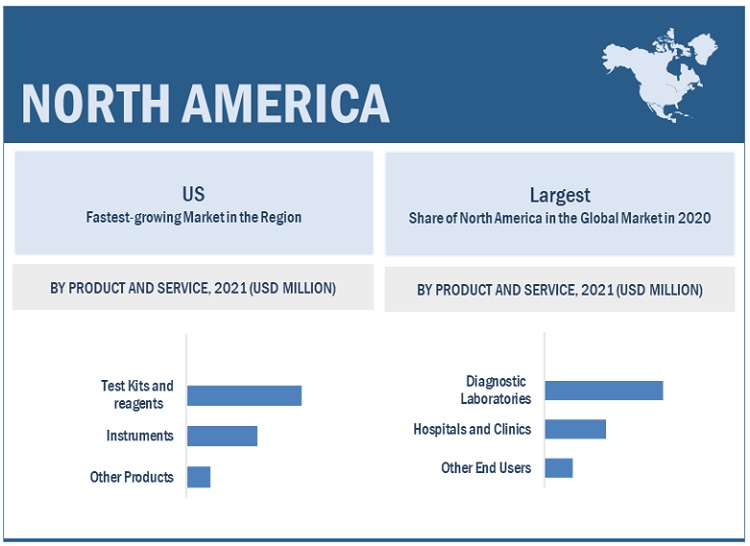
Test kits and reagents segment accounted for the largest share of the influenza diagnostics industry, by product
The influenza diagnostics market is segmented, based on product, into test kits and reagents, instruments, and other products based on product. In 2020, the test kits and reagents segment accounted for the largest share of this market, mainly due to the increasing prevalence of influenza and rising demand for rapid disease diagnosis.
Molecular diagnostic tests segment accounted for the largest share of the influenza diagnostics industry, by test type
Based on test type, the influenza diagnostics market is divided into molecular diagnostic tests and traditional diagnostic tests. In 2020, the molecular diagnostic tests segment accounted for the largest share. Factors such as growth in influenza research for diagnostic technologies and the increasing prevalence of influenza drive this market.
Diagnostic laboratories segment accounted for the largest share in the influenza diagnostics industry, by end users
The influenza diagnostics market has been segmented based on end users into diagnostic laboratories, hospitals and clinics, and other end users. In 2020, the diagnostic laboratories segment accounted for the largest share of this market. The increasing prevalence of influenza and rising demand for rapid disease diagnosis are driving the growth of this segment.
North America is the largest regional market for influenza diagnostics industry
The influenza diagnostics market is segmented into five major regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East and Africa. In 2020, North America accounted for the largest share of the global market. The North American market's growth can be attributed to the growth in influenza research for diagnostic technologies and the increasing prevalence of influenza.
To know about the assumptions considered for the study, download the pdf brochure
Some key players in the influenza diagnostics market (2021- 2026)
- Thermo Fisher Scientific, Inc. (US)
- Becton, Dickinson and Company (US)
- F. Hoffmann-LA Roche AG (Switzerland)
- Hologic, Inc. (US)
- Abbott Laboratories, Inc. (Us)
Scope of the Influenza Diagnostics Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2021 |
$0.8 billion |
|
Projected Revenue Size by 2026 |
$1.1 billion |
|
Industry Growth Rate |
Poised to grow at a CAGR of 7.7% |
|
Market Driver |
Growth in influenza research for diagnostic technologies |
|
Market Opportunity |
Advancements in genomic and proteomic technologies |
This research report categorizes the influenza diagnostics market to forecast revenue and analyze trends in each of the following submarkets:
By Product
- Test Kit and Reagents
- Instruments
- Other Products
By Test Type
-
Molecular Diagnostic Tests
- Polymerase Chain Reaction
- Isothermal Nucleic Acid Amplification Tests
- Transcription-Mediated Amplification-Based Assay
- Loop-Mediated Isothermal Amplification-Based Assay
- Nucleic Acid Sequence-Based Amplification Tests
-
Other Isothermal Nucleic Acid Amplification Tests
- Other Molecular Tests
-
Traditional Diagnostic Tests
- Rapid Influenza Diagnostic Tests
- Viral Culture Tests
- Direct Fluorescent Antibody Test
- Serological Tests
By End User
- Diagnostic Laboratories
- Hospitals and Clinics
- Other End users
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
- Latin America
- Middle East & Africa
Recent Developments of Influenza Diagnostics Industry
- In May 2021 Becton, Dickinson and Company (US) received FDA approval for the BD Veritor Plus System, which is used to detect SARS-CoV-2, influenza A, and influenza B.
- In March 2021, Abbott Laboratories, Inc. (US) received emergency use authorization (EUA) from the FDA for a laboratory PCR assay that detects and differentiates SARS-COV-2, flu A, flu B, and RSV in one test.
- In February 2021, Becton, Dickinson and Company (US) received approval from the FDA for the emergency use authorization (EUA) for a new molecular diagnostic test for both SARS-CoV-2 and influenza A+B.
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the global influenza diagnostics market?
The global influenza diagnostics market boasts a total revenue value of $1.1 billion by 2026.
What is the estimated growth rate (CAGR) of the global influenza diagnostics market?
The global influenza diagnostics market has an estimated compound annual growth rate (CAGR) of 7.7% and a revenue size in the region of $0.8 billion in 2021.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 28)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS & EXCLUSIONS OF THE STUDY
1.2.2 MARKETS COVERED
FIGURE 1 INFLUENZA DIAGNOSTICS MARKET SEGMENTATION
1.2.3 YEARS CONSIDERED FOR THE STUDY
1.3 CURRENCY
1.4 LIMITATIONS
1.5 STAKEHOLDERS
1.6 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 34)
2.1 RESEARCH DATA
2.2 RESEARCH APPROACH
FIGURE 2 INFLUENZA DIAGNOSTICS MARKET: RESEARCH DESIGN
2.2.1 SECONDARY DATA
2.2.1.1 Key data from secondary sources
2.2.2 PRIMARY DATA
2.2.2.1 Key data from primary sources
2.2.2.2 Key industry insights
FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
2.3 MARKET SIZE ESTIMATION
2.3.1 BOTTOM-UP APPROACH
FIGURE 4 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: BOTTOM-UP APPROACH
FIGURE 5 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
2.3.2 TOP-DOWN APPROACH
FIGURE 6 GLOBAL MARKET: TOP-DOWN APPROACH
2.4 MARKET BREAKDOWN & DATA TRIANGULATION
FIGURE 7 DATA TRIANGULATION METHODOLOGY
2.5 ASSUMPTIONS FOR THE STUDY
2.6 GROWTH RATE ASSUMPTIONS
2.7 LIMITATIONS
2.8 RISK ASSESSMENT
2.9 COVID-19 HEALTH ASSESSMENT
2.10 COVID-19 ECONOMIC ASSESSMENT
2.11 ASSESSMENT OF THE IMPACT OF COVID-19 ON THE ECONOMIC SCENARIO
FIGURE 8 CRITERIA IMPACTING THE GLOBAL ECONOMY
FIGURE 9 RECOVERY SCENARIO OF THE GLOBAL ECONOMY
2.12 ASSESSMENT OF THE IMPACT OF COVID-19 ON THE GLOBAL MARKET
3 EXECUTIVE SUMMARY (Page No. - 47)
FIGURE 10 INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2021 VS. 2026 (USD MILLION)
FIGURE 11 GLOBAL MARKET, BY TEST TYPE, 2021 VS. 2026 (USD MILLION)
FIGURE 12 GLOBAL MARKET, BY END USER, 2021 VS. 2026 (USD MILLION)
FIGURE 13 GLOBAL MARKET, BY REGION, 2021 VS. 2026 (USD MILLION)
4 PREMIUM INSIGHTS (Page No. - 50)
4.1 INFLUENZA DIAGNOSTICS MARKET OVERVIEW
FIGURE 14 INCREASING PREVALENCE OF INFLUENZA TO DRIVE MARKET GROWTH
4.2 GLOBAL MARKET SHARE, BY PRODUCT, 2021 VS. 2026
FIGURE 15 TEST KITS & REAGENTS WILL CONTINUE TO DOMINATE THE MARKET IN 2026
4.3 GLOBAL MARKET SHARE, BY TEST TYPE, 2021 VS. 2026
FIGURE 16 MOLECULAR DIAGNOSTIC TESTS TO DOMINATE THE MARKET DURING THE FORECAST PERIOD
4.4 GLOBAL MARKET SHARE, BY END USER, 2021 VS. 2026
FIGURE 17 DIAGNOSTIC LABORATORIES ARE THE LARGEST END USERS OF THE GLOBAL MARKET
4.5 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: GEOGRAPHIC GROWTH OPPORTUNITIES
FIGURE 18 NORTH AMERICA TO COMMAND THE LARGEST SHARE AND ASIA PACIFIC TO REGISTER THE HIGHEST GROWTH RATE IN THE GLOBAL MARKET DURING THE FORECAST PERIOD
5 MARKET OVERVIEW (Page No. - 53)
5.1 INTRODUCTION
5.2 MARKET DYNAMICS
FIGURE 19 INFLUENZA DIAGNOSTICS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
5.2.1 DRIVERS
5.2.1.1 Increasing prevalence of influenza
5.2.1.2 Growth in influenza research for diagnostic technologies
5.2.1.3 Rising demand for rapid disease diagnosis
5.2.2 RESTRAINTS
5.2.2.1 Variabilities in test sensitivity and specificity
5.2.2.2 Rising healthcare costs
5.2.3 OPPORTUNITIES
5.2.3.1 Advancements in genomic and proteomic technologies
5.2.3.2 Significant growth prospects in developing countries
5.2.4 CHALLENGES
5.2.4.1 Lack of skilled professionals
5.2.4.2 Stringent regulatory frameworks
5.3 IMPACT OF COVID-19 ON THE GLOBAL MARKET
5.4 PRICING ANALYSIS
TABLE 1 PRICES OF INFLUENZA DIAGNOSTIC PRODUCTS (2021)
5.5 PATENT ANALYSIS
5.6 TRADE ANALYSIS
5.6.1 TRADE ANALYSIS FOR DIAGNOSTIC AND LABORATORY REAGENTS
TABLE 2 IMPORT DATA FOR DIAGNOSTIC AND LABORATORY REAGENTS, BY COUNTRY, 2016–2020 (USD MILLION)
TABLE 3 EXPORT DATA FOR DIAGNOSTIC AND LABORATORY REAGENTS, BY COUNTRY, 2016–2020 (USD MILLION)
5.7 VALUE CHAIN ANALYSIS
FIGURE 20 MAJOR VALUE IS ADDED DURING THE MANUFACTURING & ASSEMBLY PHASES
5.8 SUPPLY CHAIN ANALYSIS
FIGURE 21 DISTRIBUTION—A STRATEGY PREFERRED BY PROMINENT COMPANIES
5.9 ECOSYSTEM ANALYSIS OF THE GLOBAL MARKET
FIGURE 22 GLOBAL MARKET: ECOSYSTEM ANALYSIS
5.9.1 ROLE IN THE ECOSYSTEM
5.10 PORTER’S FIVE FORCES ANALYSIS
TABLE 4 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: PORTER’S FIVE FORCES ANALYSIS
5.10.1 THREAT FROM NEW ENTRANTS
5.10.2 THREAT FROM SUBSTITUTES
5.10.3 BARGAINING POWER OF BUYERS
5.10.4 BARGAINING POWER OF SUPPLIERS
5.10.5 DEGREE OF COMPETITION
5.11 PESTLE ANALYSIS
5.12 REGULATORY LANDSCAPE
5.12.1 NORTH AMERICA
5.12.1.1 US
5.12.1.2 Canada
5.12.2 EUROPE
5.12.3 ASIA PACIFIC
5.12.3.1 Japan
5.12.3.2 India
5.12.3.3 China
5.12.4 LATIN AMERICA
5.12.4.1 Brazil
5.12.4.2 Mexico
5.12.5 MIDDLE EAST
5.12.6 AFRICA
5.13 TECHNOLOGY ANALYSIS
5.14 DISRUPTIVE TECHNOLOGIES IN THE GLOBAL MARKET
5.14.1 REVENUE SHIFT & REVENUE POCKETS FOR THE GLOBAL MARKET
FIGURE 23 REVENUE SHIFT FOR THE GLOBAL MARKET
6 INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT (Page No. - 70)
6.1 INTRODUCTION
TABLE 5 GLOBAL MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
6.2 TEST KITS & REAGENTS
6.2.1 REPEAT PURCHASE OF TEST KITS & REAGENTS TO DRIVE MARKET GROWTH IN THE COMING YEARS
FIGURE 24 INSIGHTS FROM INDUSTRY EXPERTS ON TEST KITS & REAGENTS
TABLE 6 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY FOR TEST KITS & REAGENTS, BY REGION, 2019–2026 (USD MILLION)
6.3 INSTRUMENTS
6.3.1 INCREASING NEED FOR FASTER & MORE ACCURATE TEST RESULTS TO PROPEL MARKET GROWTH
TABLE 7 KEY INSTRUMENTS IN THE GLOBAL MARKET
TABLE 8 GLOBAL MARKET FOR INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
6.4 OTHER PRODUCTS
TABLE 9 GLOBAL MARKET FOR OTHER PRODUCTS, BY REGION, 2019–2026 (USD MILLION)
7 INFLUENZA DIAGNOSTICS MARKET, BY TEST TYPE (Page No. - 76)
7.1 INTRODUCTION
TABLE 10 INFLUENZA DIAGNOSTICS MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
7.2 MOLECULAR DIAGNOSTIC TESTS
TABLE 11 MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 12 MOLECULAR DIAGNOSTIC TESTS MARKET, BY REGION,2019–2026 (USD MILLION)
7.2.1 POLYMERASE CHAIN REACTION TESTS
7.2.1.1 Ability of PCR tests to distinguish between influenza A and B viruses is driving its adoption in clinical settings
TABLE 13 POLYMERASE CHAIN REACTION TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.2.2 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS
TABLE 14 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 15 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.2.2.1 Transcription-mediated amplification-based assays
7.2.2.1.1 Wide application range in pathogen detection to drive the market growth
TABLE 16 TRANSCRIPTION-MEDIATED AMPLIFICATION-BASED ASSAYS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.2.2.2 Loop-mediated isothermal amplification-based assays
7.2.2.2.1 Rapidity, stability, and sensitivity have driven the use of loop-mediated isothermal amplification-based assays
TABLE 17 LOOP-MEDIATED ISOTHERMAL AMPLIFICATION-BASED ASSAYS MARKET,BY REGION, 2019–2026 (USD MILLION)
7.2.2.3 Nucleic acid sequence-based amplification assays
7.2.2.3.1 Nucleic acid sequence-based amplification assays are preferred in hospitals and clinical settings as they reduce the need for additional reverse transcription steps
TABLE 18 NUCLEIC ACID SEQUENCE-BASED AMPLIFICATION ASSAYS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.2.2.4 Other isothermal nucleic acid amplification tests
TABLE 19 OTHER ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.2.3 OTHER MOLECULAR DIAGNOSTIC TESTS
TABLE 20 OTHER MOLECULAR DIAGNOSTIC TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.3 TRADITIONAL DIAGNOSTIC TESTS
TABLE 21 TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 22 TRADITIONAL DIAGNOSTIC TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.3.1 RAPID INFLUENZA DIAGNOSTIC TESTS
7.3.1.1 Rapid influenza diagnostic tests are widely used due to their ease of use and rapid interpretability of results
TABLE 23 RAPID INFLUENZA DIAGNOSTIC TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.3.2 VIRAL CULTURE TESTS
7.3.2.1 Wide use of viral cultures as confirmatory tests to ensure the results of RIDTs to drive market growth
TABLE 24 VIRAL CULTURE TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.3.3 DIRECT FLUORESCENT ANTIBODY TESTS
7.3.3.1 Adoption of DFA tests is increasing due to their higher sensitivity compared to RIDTs
TABLE 25 DIRECT FLUORESCENT ANTIBODY TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
7.3.4 SEROLOGICAL TESTS
7.3.4.1 Development of lab-on-chip-based multiplex assays to support market growth
TABLE 26 SEROLOGICAL TESTS MARKET, BY REGION, 2019–2026 (USD MILLION)
8 INFLUENZA DIAGNOSTICS MARKET, BY END USER (Page No. - 89)
8.1 INTRODUCTION
TABLE 27 GLOBAL MARKET, BY END USER, 2019–2026 (USD MILLION)
8.2 DIAGNOSTIC LABORATORIES
8.2.1 LARGE NUMBER OF INFLUENZA DIAGNOSTIC TESTS PERFORMED IN DIAGNOSTIC LABORATORIES TO DRIVE MARKET GROWTH
TABLE 28 GLOBAL MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2019–2026 (USD MILLION)
8.3 HOSPITALS & CLINICS
8.3.1 GROWING GLOBAL NUMBER OF HOSPITALS DUE TO INCREASING INFECTIOUS DISEASE INCIDENCE TO DRIVE MARKET GROWTH
TABLE 29 GLOBAL MARKET FOR HOSPITALS & CLINICS, BY REGION, 2019–2026 (USD MILLION)
8.4 OTHER END USERS
TABLE 30 GLOBAL MARKET FOR OTHER END USERS, BY REGION, 2019–2026 (USD MILLION)
9 INFLUENZA DIAGNOSTICS MARKET, BY REGION (Page No. - 93)
9.1 INTRODUCTION
TABLE 31 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY, BY REGION, 2019–2026 (USD MILLION)
TABLE 32 GLOBAL MARKET, BY REGION, 2019–2026 (MILLION TESTS)
9.2 NORTH AMERICA
FIGURE 25 NORTH AMERICA: INFLUENZA DIAGNOSTICS MARKET SNAPSHOT
TABLE 33 NORTH AMERICA: MARKET, BY COUNTRY, 2019–2026 (USD MILLION)
TABLE 34 NORTH AMERICA: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 35 NORTH AMERICA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 36 NORTH AMERICA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 37 NORTH AMERICA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 38 NORTH AMERICA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 39 NORTH AMERICA: MARKET, BY END USER,2019–2026 (USD MILLION)
9.2.1 US
9.2.1.1 Technological advancements in influenza diagnostics to favor market growth in the US
TABLE 40 US: PRODUCT APPROVALS IN THE INFLUENZA DIAGNOSTICS MARKET
TABLE 41 US: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 42 US: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 43 US: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 44 US: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 45 US: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 46 US: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.2.2 CANADA
9.2.2.1 Government initiatives for developing diagnostic techniques to support market growth in Canada
TABLE 47 CANADA: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 48 CANADA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 49 CANADA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 50 CANADA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 51 CANADA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 52 CANADA: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.3 EUROPE
TABLE 53 EUROPE: INFLUENZA DIAGNOSTICS MARKET, BY COUNTRY,2019–2026 (USD MILLION)
TABLE 54 EUROPE: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 55 EUROPE: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 56 EUROPE: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 57 EUROPE: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 58 EUROPE: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 59 EUROPE: INFLUENZA DIAGNOSTICS INDUSTRY, BY END USER, 2019–2026 (USD MILLION)
9.3.1 GERMANY
9.3.1.1 Increasing demand for technologically advanced diagnostic techniques to drive market growth in Germany
TABLE 60 GERMANY: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 61 GERMANY: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 62 GERMANY: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 63 GERMANY: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 64 GERMANY: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 65 GERMANY: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.3.2 ITALY
9.3.2.1 Well-developed healthcare system in Italy to drive the adoption of advanced diagnostic technologies
TABLE 66 ITALY: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 67 ITALY: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 68 ITALY: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 69 ITALY: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 70 ITALY: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 71 ITALY: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.3.3 FRANCE
9.3.3.1 High healthcare expenditure by the government to drive market growth in France
TABLE 72 FRANCE: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 73 FRANCE: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 74 FRANCE: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 75 FRANCE: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 76 FRANCE: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 77 FRANCE: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.3.4 SPAIN
9.3.4.1 Healthcare infrastructural improvements indicate favorable prospects for market growth in Spain
TABLE 78 SPAIN: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 79 SPAIN: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 80 SPAIN: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 81 SPAIN: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 82 SPAIN: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 83 SPAIN: INFLUENZA DIAGNOSTICS INDUSTRY, BY END USER, 2019–2026 (USD MILLION)
9.3.5 UK
9.3.5.1 Frequent influenza outbreaks in the UK to boost the growth of the influenza diagnostics market
TABLE 84 UK: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 85 UK: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 86 UK: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 87 UK: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 88 UK: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 89 UK: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.3.6 REST OF EUROPE
TABLE 90 ROE: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 91 ROE: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 92 ROE: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 93 ROE: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 94 ROE: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 95 ROE: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.4 ASIA PACIFIC
FIGURE 26 ASIA PACIFIC: INFLUENZA DIAGNOSTICS MARKET SNAPSHOT
TABLE 96 ASIA PACIFIC: MARKET, BY COUNTRY, 2019–2026 (USD MILLION)
TABLE 97 ASIA PACIFIC: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 98 ASIA PACIFIC: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 99 ASIA PACIFIC: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 100 ASIA PACIFIC: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 101 ASIA PACIFIC: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 102 ASIA PACIFIC: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.4.1 CHINA
9.4.1.1 Growing investments by government agencies to drive market growth in China
TABLE 103 CHINA: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 104 CHINA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 105 CHINA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 106 CHINA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 107 CHINA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 108 CHINA: INFLUENZA DIAGNOSTICS INDUSTRY, BY END USER, 2019–2026 (USD MILLION)
9.4.2 JAPAN
9.4.2.1 Universal healthcare reimbursement policy to drive market growth in Japan
TABLE 109 JAPAN: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 110 JAPAN: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 111 JAPAN: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 112 JAPAN: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 113 JAPAN: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE,2019–2026 (USD MILLION)
TABLE 114 JAPAN: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.4.3 INDIA
9.4.3.1 Increase in public and private investments in the country’s healthcare system will drive market growth
TABLE 115 INDIA: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 116 INDIA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 117 INDIA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 118 INDIA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 119 INDIA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 120 INDIA: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.4.4 REST OF ASIA PACIFIC
TABLE 121 ROAPAC: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 122 ROAPAC: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 123 ROAPAC: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 124 ROAPAC: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 125 ROAPAC: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 126 ROAPAC: MARKET, BY END USER, 2019–2026 (USD MILLION)
9.5 LATIN AMERICA
9.5.1 RISING DISEASE PREVALENCE AND INCREASING HEALTHCARE EXPENDITURE TO DRIVE THE LATAM MARKET
TABLE 127 LATIN AMERICA: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 128 LATIN AMERICA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 129 LATIN AMERICA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 130 LATIN AMERICA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 131 LATIN AMERICA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 132 LATIN AMERICA: INFLUENZA DIAGNOSTICS INDUSTRY, BY END USER, 2019–2026 (USD MILLION)
9.6 MIDDLE EAST & AFRICA
9.6.1 RISING ACCESS TO ADVANCED TECHNOLOGIES AND GROWING INVESTMENTS WILL SUPPORT MARKET GROWTH
TABLE 133 MIDDLE EAST & AFRICA: INFLUENZA DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 134 MIDDLE EAST & AFRICA: MARKET, BY TEST TYPE, 2019–2026 (USD MILLION)
TABLE 135 MIDDLE EAST & AFRICA: MOLECULAR DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 136 MIDDLE EAST & AFRICA: ISOTHERMAL NUCLEIC ACID AMPLIFICATION TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 137 MIDDLE EAST & AFRICA: TRADITIONAL DIAGNOSTIC TESTS MARKET, BY TYPE, 2019–2026 (USD MILLION)
TABLE 138 MIDDLE EAST & AFRICA: INFLUENZA DIAGNOSTICS INDUSTRY, BY END USER, 2019–2026 (USD MILLION)
10 COMPETITIVE LANDSCAPE (Page No. - 139)
10.1 OVERVIEW
10.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
10.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN THE INFLUENZA DIAGNOSTICS MARKET
TABLE 139 STRATEGIES ADOPTED BY KEY INFLUENZA DIAGNOSTIC PRODUCT MANUFACTURING COMPANIES
10.3 REVENUE SHARE ANALYSIS OF THE TOP MARKET PLAYERS
FIGURE 27 REVENUE SHARE ANALYSIS OF THE TOP PLAYERS IN THE GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY
10.4 MARKET SHARE ANALYSIS
FIGURE 28 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY SHARE, BY KEY PLAYER (2020)
TABLE 140 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: DEGREE OF COMPETITION
10.5 COMPANY EVALUATION QUADRANT
10.5.1 STARS
10.5.2 EMERGING LEADERS
10.5.3 PERVASIVE PLAYERS
10.5.4 PARTICIPANTS
FIGURE 29 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: COMPANY EVALUATION QUADRANT, 2020
10.6 COMPANY EVALUATION QUADRANT FOR STARTUPS/SMES (2020)
10.6.1 PROGRESSIVE COMPANIES
10.6.2 STARTING BLOCKS
10.6.3 RESPONSIVE COMPANIES
10.6.4 DYNAMIC COMPANIES
FIGURE 30 GLOBAL INFLUENZA DIAGNOSTICS INDUSTRY: COMPANY EVALUATION QUADRANT FOR START-UPS/SMES, 2020
10.7 COMPETITIVE BENCHMARKING
FIGURE 31 PRODUCT AND GEOGRAPHIC FOOTPRINT ANALYSIS OF THE TOP PLAYERS IN THE INFLUENZA DIAGNOSTICS MARKET
TABLE 141 COMPANY PRODUCT FOOTPRINT
TABLE 142 COMPANY REGIONAL FOOTPRINT
10.8 COMPETITIVE SCENARIO
10.8.1 PRODUCT LAUNCHES & APPROVALS
TABLE 143 KEY PRODUCT LAUNCHES & APPROVALS
10.8.2 DEALS
TABLE 144 KEY DEALS
11 COMPANY PROFILES (Page No. - 150)
11.1 KEY PLAYERS
(Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))*
11.1.1 THERMO FISHER SCIENTIFIC, INC.
TABLE 145 THERMO FISHER SCIENTIFIC, INC.: BUSINESS OVERVIEW
FIGURE 32 THERMO FISHER SCIENTIFIC, INC.: COMPANY SNAPSHOT (2020)
11.1.2 BECTON, DICKINSON AND COMPANY
TABLE 146 BECTON, DICKINSON AND COMPANY: BUSINESS OVERVIEW
FIGURE 33 BECTON, DICKINSON AND COMPANY: COMPANY SNAPSHOT (2020)
11.1.3 F. HOFFMANN-LA ROCHE AG
TABLE 147 F. HOFFMANN-LA ROCHE AG: BUSINESS OVERVIEW
FIGURE 34 F. HOFFMANN-LA ROCHE AG: COMPANY SNAPSHOT (2020)
11.1.4 HOLOGIC, INC.
TABLE 148 HOLOGIC, INC.: BUSINESS OVERVIEW
FIGURE 35 HOLOGIC, INC.: COMPANY SNAPSHOT (2020)
11.1.5 ABBOTT LABORATORIES, INC.
TABLE 149 ABBOTT LABORATORIES, INC.: BUSINESS OVERVIEW
FIGURE 36 ABBOTT LABORATORIES, INC.: COMPANY SNAPSHOT (2020)
11.1.6 DANAHER CORPORATION
TABLE 150 DANAHER CORPORATION: BUSINESS OVERVIEW
FIGURE 37 DANAHER CORPORATION: COMPANY SNAPSHOT (2020)
11.1.7 QUIDEL CORPORATION
TABLE 151 QUIDEL CORPORATION: BUSINESS OVERVIEW
FIGURE 38 QUIDEL CORPORATION: COMPANY SNAPSHOT (2020)
11.1.8 SIEMENS HEALTHINEERS
TABLE 152 SIEMENS HEALTHINEERS: BUSINESS OVERVIEW
FIGURE 39 SIEMENS HEALTHINEERS: COMPANY SNAPSHOT (2020)
11.1.9 BIOMÉRIEUX SA
TABLE 153 BIOMÉRIEUX SA: BUSINESS OVERVIEW
FIGURE 40 BIOMÉRIEUX SA: COMPANY SNAPSHOT (2020)
11.1.10 MERIDIAN BIOSCIENCE
TABLE 154 MERIDIAN BIOSCIENCE: BUSINESS OVERVIEW
FIGURE 41 MERIDIAN BIOSCIENCE: COMPANY SNAPSHOT (2020)
11.2 OTHER PLAYERS
11.2.1 GENMARK DIAGNOSTICS, INC.
11.2.2 LUMINEX CORPORATION
11.2.3 TECAN TRADING AG
11.2.4 DIASORIN SA
11.2.5 ALTONA DIAGNOSTICS GMBH
11.2.6 SEKISUI DIAGNOSTICS
11.2.7 SA SCIENTIFIC, LTD.
11.2.8 CORIS BIOCONCEPT SPRL
11.2.9 ELITECH GROUP
11.2.10 MAST GROUP LTD.
11.2.11 GENOME DIAGNOSTICS PVT. LTD.
11.2.12 GERMAINE LABORATORIES, INC.
11.2.13 RESPONSE BIOMEDICAL CORP.
11.2.14 TAUNS LABORATORIES, INC.
11.2.15 3B BLACKBIO BIOTECH INDIA LTD.
*Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
12 APPENDIX (Page No. - 187)
12.1 INSIGHTS FROM INDUSTRY EXPERTS
12.2 DISCUSSION GUIDE
12.3 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
12.4 AVAILABLE CUSTOMIZATIONS
12.5 RELATED REPORTS
12.6 AUTHOR DETAILS
This study involved four major activities in estimating the current size of the influenza diagnostics market. Exhaustive secondary research was carried out to collect information on the market, its peer markets, and its parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate segments and subsegments' market size.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
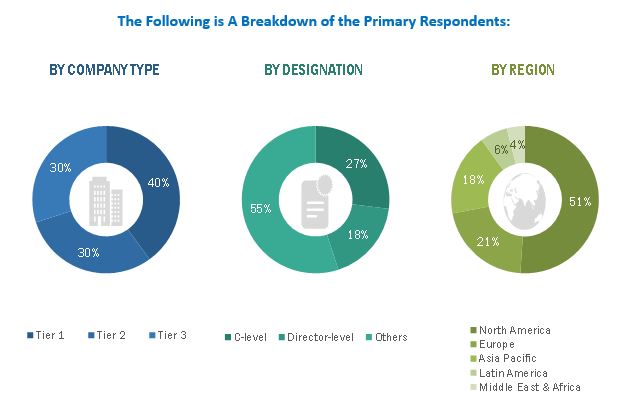
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify the critical qualitative and quantitative information as well as assess future prospects.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the influenza diagnostics market's total size. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry have been identified through extensive secondary research
- The revenues generated by leading players operating in the influenza diagnostics market have been determined through primary and secondary research
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Top-Down Approach

Data Triangulation
After arriving at the overall market size applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Report Objectives
- To define, describe, segment, and forecast the influenza diagnostics market by product, test type, end user, and region.
- To provide detailed information regarding the major factors influencing the market growth (drivers, restraints, opportunities, and challenges).
- To analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall market.
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players.
- To forecast the size of the market segments with respect to five regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa,
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies2.
- To track and analyze company developments such as acquisition, approval and product launch in the influenza diagnostics market.
- To benchmark players within the market using the proprietary “Competitive Leadership Mapping” framework, which analyzes market players on various parameters within the broad categories of business and product strategy.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Country Information
- Influenza diagnostics market size and growth rate estimates for countries in the Rest of Europe, the Rest of Asia Pacific, Latin America, and Middle East & Africa.
Company Profiles
- Product portfolio matrix for leading market players.




 Generating Response ...
Generating Response ...











Growth opportunities and latent adjacency in Influenza Diagnostics Market
Which factors feeds the global growth of Influenza Diagnostics Market?
Which of the geographical segment is most suitable for the major growth in the Influenza Diagnostics Market?
I want to know more about the target audience for this report. Thank you