This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global precision diagnostics and medicine market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The market for the companies offering precision medicines and diagnostic kits is arrived at by secondary data available through paid and unpaid sources, analyzing the product portfolios of the major companies in the ecosystem, and rating the companies by their performance and quality. Various sources were referred to in the secondary research process to identify and collect information for this study. The secondary sources include annual reports, press releases, investor presentations of companies, white papers, journals, certified publications, and articles from recognized authors, directories, and databases. The secondary research was used to obtain critical information on the industry’s value chain, the total pool of key players, market classification, and segmentation from the market and technology-oriented perspectives. Secondary data was analyzed to arrive at the overall size of the global precision medicine market, which was validated through primary research.
Primary Research
Extensive primary research was conducted after acquiring basic knowledge about the global precision diagnostics and medicine market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side, such as hospitals and clinics, and experts from the supply side, such as C-level and D-level executives, product managers, marketing & sales managers of key manufacturers, distributors, and channel partners. These interviews were conducted across major regions, namely, North America, Europe, the Asia Pacific, and the Rest of the World (including Latin America, the Middle East, and Africa). This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
In the complete market engineering process, the top-down and bottom-up approaches were extensively used, along with several data triangulation methods, to perform the market estimation and market forecasting for the overall market segments and subsegments listed in this report. Extensive qualitative and quantitative analysis was performed on the complete market engineering process to list the key information/insights throughout the report.
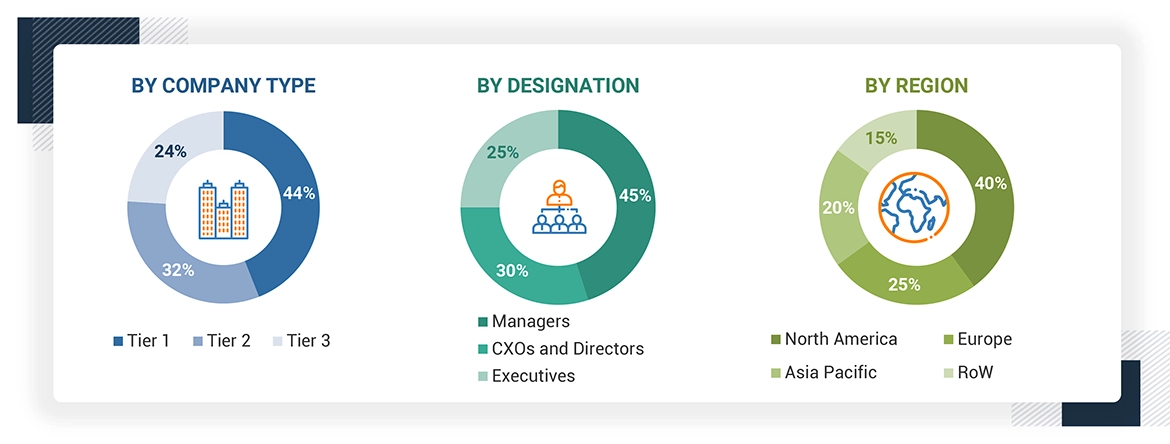
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The global size of the precision diagnostics and medicine market is a sum of the precision diagnostics and precision medicine market. Both these markets were estimated and validated through multiple approaches such as the top-down and bottom-up approaches. These methods were also used extensively to estimate the size of various subsegments in the markets. The research methodology used to estimate the market size includes the following:
The key players in the precision diagnostics and precision medicine market have been identified through extensive primary and secondary research.
The revenues generated from the precision diagnostics and precision medicine market businesses of leading players have been determined respectively through primary and secondary research.
All percentage shares, splits, and breakdowns for respective markets have been determined using secondary sources and verified through primary sources.
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable.
Market Definition
Precision medicine, also known as ‘personalized medicine’, is an innovative approach to customizing treatment plans that considers the differences in an individual’s genes, environment, and lifestyle. The precision medicine market includes both precision therapeutics and precision diagnostics products. Precision diagnostics products are tests used to diagnose diseases more accurately by analyzing genetic and molecular information. Precision therapeutics, on the other hand, include drugs that are designed to target specific genetic or molecular features of a disease, offering personalized treatments for conditions like cancer, rare diseases, and infections.
Stakeholders
-
Pharmaceutical Companies
-
Biotechnology Companies
-
Research Institutions and Academic Centers
-
Regulatory Agencies
-
Health Insurers and Payers
-
Private & government-funding organizations
Report Objectives
-
To define, describe, and forecast the precision diagnostics and medicine market based on diagnostic solution type, diagnostic solution indication, diagnostic solution end users, therapeutic solution products, therapeutic solution indication, therapeutic solution end users, and region
-
To provide detailed information regarding the major factors influencing the market growth, such as drivers, restraints, opportunities, and challenges
-
To strategically analyze the micromarkets with respect to individual growth trends, future prospects, and contributions to the overall precision medicine market
-
To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
-
To analyze subsegments of the market with respect to individual growth trends, prospects, and contributions to the overall market
-
To forecast the revenue of the market segments with respect to five main regions, namely, North America (US and Canada), Europe (Germany, UK, France, Italy, Spain, and the Rest of Europe), Asia Pacific (China, Japan, India, Australia, and the Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America, and the Middle East & Africa.
-
To profile the key market players and comprehensively analyze their market shares and core competencies2
-
To track and analyze competitive developments such as product launches and approvals, agreements, partnerships, acquisitions, business expansions, and research & development activities in the precision diagnostics and medicine market.



Growth opportunities and latent adjacency in Precision Diagnostics & Medicine Market