2
RESEARCH METHODOLOGY
47
5
MARKET OVERVIEW
Navigate market shifts as biologic drug complexity and generics disrupt pricing and production.
71
5.2.1.1
GLP-1 CAPACITY CRUNCH
5.2.1.2
INCREASING DEVELOPMENT AND MANUFACTURING OF ANTIBODY DRUG CONJUGATES
5.2.1.3
LOSS OF EXCLUSIVITY OF BLOCKBUSTER BIOLOGICS
5.2.1.4
INCREASING COMPLEXITY OF INJECTABLE DRUG FORMATS
5.2.2.1
PRICING PRESSURE ON INNOVATOR DRUGS
5.2.2.2
GROWING PENETRATION OF GENERICS AND BIOSIMILARS
5.2.2.3
STRICT REGULATORY COMPLIANCE
5.2.2.4
RISK IN ADVANCED THERAPIES PIPELINE
5.2.3.1
RISING DEMAND FOR CELL AND GENE THERAPIES
5.2.3.2
GROWING INCLINATION TOWARD ONE-STOP-SHOP MODEL
5.2.3.3
MARKET EXPANSION IN EMERGING COUNTRIES
5.2.3.4
BOOMING RADIOPHARMACEUTICAL AND NUCLEAR MEDICINE SEGMENT
5.2.4.1
GLOBAL TRADE INSTABILITY AND INSOURCING
5.3
TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
5.4.1
INDICATIVE PRICING ANALYSIS, BY SERVICE
5.4.2
INDICATIVE PRICING ANALYSIS, BY REGION
5.4.3
COMPARISON OF CDMO PRICING MODELS
5.6
SUPPLY CHAIN ANALYSIS
5.7.1
MANUFACTURING SITE, EXPANSION, AND CAPEX TREND OF KEY CDMOS
5.8
INVESTMENT AND FUNDING SCENARIO
5.9.1.1
SINGLE-USE BIOPROCESSING SYSTEMS
5.9.1.2
CONTINUOUS MANUFACTURING
5.9.1.3
ADVANCED FORMULATION TECHNOLOGIES
5.9.2
COMPLEMENTARY TECHNOLOGIES
5.9.2.1
HIGH-PERFORMANCE LIQUID CHROMATOGRAPHY
5.9.2.2
MASS SPECTROMETRY
5.9.2.3
NEXT-GENERATION SEQUENCING
5.9.2.4
AUTOMATION AND ROBOTICS
5.9.2.5
PROCESS ANALYTICAL TECHNOLOGY
5.9.3
ADJACENT TECHNOLOGIES
5.9.3.2
ARTIFICIAL INTELLIGENCE AND MACHINE LEARNING
5.11
KEY CONFERENCES AND EVENTS, 2025–2026
5.12
REGULATORY LANDSCAPE
5.12.1
REGULATORY SCENARIO
5.12.2
REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.13
PORTER’S FIVE FORCES ANALYSIS
5.13.1
BARGAINING POWER OF SUPPLIERS
5.13.2
BARGAINING POWER OF BUYERS
5.13.3
THREAT OF NEW ENTRANTS
5.13.4
THREAT OF SUBSTITUTES
5.13.5
INTENSITY OF COMPETITIVE RIVALRY
5.14
KEY STAKEHOLDERS AND BUYING CRITERIA
5.14.1
KEY STAKEHOLDERS IN BUYING PROCESS
5.15
UNMET NEEDS OF END USERS
5.15.1
CAPACITY AND TIMELY ACCESS
5.15.2
COST EFFICIENCY AND FLEXIBILITY
5.15.3
END-TO-END INTEGRATED SERVICES
5.15.4
ADVANCED MODALITY EXPERTISE
5.15.5
QUALITY AND REGULATORY CONSISTENCY
5.16
ABBREVIATED NEW DRUG APPLICATION (ANDA) APPROVALS
5.17
IMPACT OF AI/GEN AI ON PHARMACEUTICAL CONTRACT MANUFACTURING MARKET
5.17.2
MARKET POTENTIAL OF AI/GEN AI
5.17.4
KEY COMPANIES IMPLEMENTING AI/GEN AI
5.18
IMPACT OF 2025 US TARIFFS ON PHARMACEUTICAL CONTRACT MANUFACTURING MARKET
5.18.3
PRICE IMPACT ANALYSIS
5.18.4
IMPACT ON COUNTRY/REGION
5.18.5
IMPACT ON END-USE INDUSTRIES
6
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE
Market Size & Growth Rate Forecast Analysis to 2030 in USD Billion | 116 Data Tables
126
6.2
PHARMACEUTICAL MANUFACTURING SERVICES
6.2.1
PHARMACEUTICAL API MANUFACTURING SERVICES
6.2.1.1
RISING CASES OF PATENT EXPIRY TO FUEL MARKET
6.2.2
PHARMACEUTICAL FDF MANUFACTURING SERVICES
6.2.2.1
PARENTERAL MANUFACTURING SERVICES
6.2.2.2
TABLET MANUFACTURING SERVICES
6.2.2.3
CAPSULE MANUFACTURING SERVICES
6.2.2.4
ORAL LIQUID MANUFACTURING SERVICES
6.2.2.5
SEMI-SOLID MANUFACTURING SERVICES
6.2.2.6
OTHER FDF MANUFACTURING SERVICES
6.3
DRUG DEVELOPMENT SERVICES
6.3.1
INCREASING COST OF DRUG DISCOVERY AND DEVELOPMENT TO EXPEDITE GROWTH
6.4
BIOLOGICS MANUFACTURING SERVICES
6.4.1
BIOLOGICS API MANUFACTURING SERVICES
6.4.1.1
RISING INVESTMENTS IN SINGLE-USE PRODUCTION CAPACITIES TO BOLSTER GROWTH
6.4.2
BIOLOGICS FDF MANUFACTURING SERVICES
6.4.2.1
GROWING DEMAND FOR GENERIC MEDICINES AND BIOLOGICS TO FUEL MARKET
6.5
PACKAGING & LABELING SERVICES
6.5.1
INCREASING COMPLEXITY AND STRINGENCY OF REGULATORY REQUIREMENTS FOR DRUG SAFETY AND EFFICACY TO FAVOR GROWTH
6.6.1
SURGE IN DEMAND FOR BIOLOGICS AND COMPLEX INJECTABLE THERAPIES TO BOOST MARKET
7
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE
Market Size & Growth Rate Forecast Analysis to 2030 in USD Billion | 87 Data Tables
185
7.2.1
HIGH-POTENCY SMALL MOLECULES
7.2.1.1
INCREASING ADOPTION OF HIGH-POTENCY SMALL MOLECULES FOR TREATING ONCOLOGY, RARE DISEASES, AND OTHER DISEASES TO AID GROWTH
7.2.2
OLIGONUCLEOTIDE & SYNTHETIC PEPTIDES
7.2.2.1
GROWING FOCUS ON NEXT-GEN THERAPIES TO DRIVE MARKET
7.2.3
RADIOPHARMACEUTICAL MOLECULES
7.2.3.1
ADVANCES IN ISOTOPE PRODUCTION, RADIOLABELING CHEMISTRY, AND THERANOSTIC APPROACHES TO BOOST MARKET
7.2.4
OTHER SMALL MOLECULES
7.3.1
MONOCLONAL ANTIBODIES
7.3.1.1
RISING USE OF MABS IN MANAGING CANCER, AUTOIMMUNE DISORDERS, AND OSTEOPOROSIS TO SPUR GROWTH
7.3.2
CELL & GENE THERAPIES
7.3.2.1
EMERGING TREATMENTS FOR PREVIOUSLY UNTREATABLE GENETIC AND RARE DISEASES TO BOOST MARKET
7.3.3
ANTIBODY DRUG CONJUGATES
7.3.3.1
GROWING NEED FOR PRECISION MEDICINES TO FUEL MARKET
7.3.4.1
GROWING THREAT OF INFECTIOUS DISEASE OUTBREAKS TO DRIVE MARKET
7.3.5
THERAPEUTIC PEPTIDES & PROTEINS
7.3.5.1
INCREASING RESEARCH IN GENOMICS TO SUPPORT GROWTH
7.3.6
OTHER LARGE MOLECULES
8
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER
Market Size & Growth Rate Forecast Analysis to 2030 in USD Billion | 29 Data Tables
229
8.2
BIG PHARMACEUTICAL COMPANIES
8.2.1
PRICING PRESSURE IN PHARMACEUTICAL INDUSTRY TO CONTRIBUTE TO GROWTH
8.3
SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES
8.3.1
NEED TO SAVE IN-HOUSE DRUG MANUFACTURING COST TO FOSTER GROWTH
8.4
GENERIC PHARMACEUTICAL COMPANIES
8.4.1
FAVORABLE GOVERNMENT SUPPORT FOR ADOPTION OF GENERIC DRUGS TO PROPEL MARKET
9
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY REGION
Comprehensive coverage of 7 Regions with country-level deep-dive of 17 Countries | 253 Data Tables.
245
9.2.1
MACROECONOMIC OUTLOOK FOR NORTH AMERICA
9.2.2.1
HIGH CLINICAL TRIAL AND DRUG DISCOVERY ACTIVITIES TO FOSTER GROWTH
9.2.3.1
FAVORABLE GOVERNMENT SUPPORT AND INCREASED FOCUS ON R&D ACTIVITIES TO SUSTAIN GROWTH
9.3.1
MACROECONOMIC OUTLOOK FOR EUROPE
9.3.2.1
FREE DRUG-PRICING POLICY AND HIGH PHARMACEUTICAL INVESTMENTS TO SPEED UP GROWTH
9.3.3.1
FAVORABLE R&D FUNDING SCENARIO TO AUGMENT GROWTH
9.3.4.1
BOOMING LIFE SCIENCE RESEARCH AND GROWING GENERICS MARKET TO DRIVE MARKET
9.3.5.1
RISING COMMERCIAL DRUG DEVELOPMENT AND INCREASING LIFE SCIENCE R&D TO FAVOR GROWTH
9.3.6.1
RAPID GROWTH AS GLOBAL PHARMACEUTICAL MANUFACTURING HUB TO DRIVE MARKET
9.3.7.1
UNIVERSAL HEALTH COVERAGE AND ADVANCED GOVERNMENT-SUPPORTED MEDICAL INFRASTRUCTURE TO ENCOURAGE GROWTH
9.3.8.1
GROWING PHARMACEUTICAL R&D EXPENDITURE AND INCREASING OUTSOURCING OF TRIAL PHASES TO BOOST MARKET
9.4.1
MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
9.4.2.1
MADE IN CHINA 2025 INITIATIVE AND HIGH GOVERNMENT DRUG DEVELOPMENT INVESTMENTS TO AID GROWTH
9.4.3.1
HIGH GERIATRIC POPULATION AND ADVANCED PHARMACEUTICAL RESEARCH TO AUGMENT GROWTH
9.4.4.1
INCREASED BIOTECH INVESTMENTS AND DEVELOPED PHARMA SECTOR TO PROPEL MARKET
9.4.5.1
FAVORABLE INDUSTRIAL ENVIRONMENT AND LOWER LABOR COSTS TO DRIVE MARKET
9.4.6.1
STRATEGIC GEOGRAPHICAL LOCATION AND STRINGENT REGULATORY ENVIRONMENT TO FOSTER GROWTH
9.4.7
REST OF ASIA PACIFIC
9.5.1
MACROECONOMIC OUTLOOK FOR LATIN AMERICA
9.5.2.1
RAPID PATIENT ENROLMENT AND INCREASED FOCUS ON ADVANCED CLINICAL TRIALS TO EXPEDITE GROWTH
9.5.3.1
SURGE IN PHARMACEUTICAL GOODS EXPORT TO PROMOTE GROWTH
9.5.4
REST OF LATIN AMERICA
9.6.1
MACROECONOMIC OUTLOOK FOR MIDDLE EAST
9.6.2.1
KINGDOM OF SAUDI ARABIA (KSA)
9.6.2.3
REST OF GCC COUNTRIES
9.6.3
REST OF MIDDLE EAST
9.7.1
RISING PREVALENCE OF CHRONIC DISEASES TO FACILITATE GROWTH
9.7.2
MACROECONOMIC OUTLOOK OF AFRICA
10
COMPETITIVE LANDSCAPE
Discover the strategic moves defining market leadership in the evolving competitive landscape.
391
10.2
KEY PLAYER STRATEGIES/RIGHT TO WIN
10.3
REVENUE SHARE ANALYSIS, 2020–2024
10.4
MARKET SHARE ANALYSIS, 2024
10.5
COMPANY VALUATION AND FINANCIAL METRICS
10.6
BRAND/SERVICE COMPARISON
10.6.1
THERMO FISHER SCIENTIFIC INC.
10.6.3
WUXI BIOLOGICS AND WUXI APPTEC
10.7
COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
10.7.5
COMPANY FOOTPRINT: KEY PLAYERS, 2024
10.7.5.1
COMPANY FOOTPRINT
10.7.5.2
REGION FOOTPRINT
10.7.5.3
SERVICE FOOTPRINT
10.7.5.4
MOLECULE FOOTPRINT
10.8
COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
10.8.1
PROGRESSIVE COMPANIES
10.8.2
RESPONSIVE COMPANIES
10.8.5
COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
10.8.5.1
DETAILED LIST OF KEY STARTUPS/SMES
10.8.5.2
COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
10.9
COMPETITIVE SCENARIO
11
COMPANY PROFILES
In-depth Company Profiles of Leading Market Players with detailed Business Overview, Product and Service Portfolio, Recent Developments, and Unique Analyst Perspective (MnM View)
419
11.1.1
THERMO FISHER SCIENTIFIC INC.
11.1.1.1
BUSINESS OVERVIEW
11.1.1.2
SERVICES OFFERED
11.1.1.3
RECENT DEVELOPMENTS
11.1.7
BOEHRINGER INGELHEIM INTERNATIONAL GMBH
11.1.9
FUJIFILM HOLDINGS CORPORATION
11.1.11
SIEGFRIED HOLDING AG
11.1.14
CHARLES RIVER LABORATORIES
11.1.17
ALCAMI CORPORATION
11.1.18
EUROFINS SCIENTIFIC
11.2.1
PIRAMAL PHARMA SOLUTIONS
11.2.2
SYNGENE INTERNATIONAL LIMITED
11.2.3
CAMBREX CORPORATION
11.2.4
JUBILANT PHARMANOVA LIMITED
11.2.6
PIERRE FABRE LABORATORIES
11.2.10
SHARP SERVICES, LLC
11.2.11
GRAND RIVER ASEPTIC MANUFACTURING
11.2.13
PCI PHARMA SERVICES
11.2.14
CURIA GLOBAL, INC.
11.2.15
SIMTRA BIOPHARMA SOLUTIONS
11.2.17
MABPLEX INTERNATIONAL CO. LTD.
12.2
KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
12.3
CUSTOMIZATION OPTIONS
TABLE 1
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: INCLUSIONS AND EXCLUSIONS
TABLE 2
IMPACT ANALYSIS OF SUPPLY-SIDE AND DEMAND-SIDE FACTORS
TABLE 3
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: RISK ANALYSIS
TABLE 4
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: IMPACT ANALYSIS OF MARKET DYNAMICS
TABLE 5
GLP-1 PIPELINE, EXPECTED LAUNCH BY 2030
TABLE 6
ADC PIPELINE, EXPECTED LAUNCH BY 2030
TABLE 7
IMPENDING AND ONGOING PATENT EXPIRIES OF BLOCKBUSTER BIOLOGICS, 2023–2035
TABLE 8
LIST OF RECENTLY APPROVED INJECTABLES, 2022–2024
TABLE 9
CELL & GENE THERAPY-SPECIFIC CDMO EXPANSIONS, 2023–2025
TABLE 10
CELL & GENE THERAPY PIPELINE, EXPECTED LAUNCH BY 2030
TABLE 11
INDICATIVE PRICING ANALYSIS, BY SERVICE
TABLE 12
INDICATIVE PRICING ANALYSIS, BY REGION
TABLE 13
PRICING MODELS FOR CDMO SERVICES
TABLE 14
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: ROLE OF COMPANIES IN SUPPLY CHAIN
TABLE 15
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: ROLE OF COMPANIES IN ECOSYSTEM
TABLE 16
MANUFACTURING SITES OF KEY CDMOS
TABLE 17
LIST OF SITE EXPANSIONS FOR KEY CDMOS, 2023–2025
TABLE 18
CAPEX FOR SELECTED PUBLICLY LISTED KEY CDMOS, 2021–2024 (USD MILLION)
TABLE 19
NUMBER OF PATENTS FILED IN PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, 2014–2024
TABLE 20
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: KEY CONFERENCES AND EVENTS, 2025–2026
TABLE 21
REGULATORY SCENARIO, BY COUNTRY
TABLE 22
NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 23
EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 24
ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 25
LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 26
MIDDLE EAST: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 27
AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 28
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: PORTER’S FIVE FORCES ANALYSIS
TABLE 29
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF PHARMACEUTICAL CONTRACT MANUFACTURING SERVICES (%)
TABLE 30
BUYING CRITERIA FOR PHARMACEUTICAL CONTRACT MANUFACTURING, BY END USER
TABLE 31
US-ADJUSTED RECIPROCAL TARIFF RATES
TABLE 32
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 33
PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 34
PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 35
NORTH AMERICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 36
EUROPE: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 37
ASIA PACIFIC: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 38
LATIN AMERICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 39
MIDDLE EAST: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 40
GCC COUNTRIES: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 41
PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030(USD BILLION)
TABLE 42
NORTH AMERICA: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 43
EUROPE: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 44
ASIA PACIFIC: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 45
LATIN AMERICA: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 46
MIDDLE EAST: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030(USD BILLION)
TABLE 47
GCC COUNTRIES: PHARMACEUTICAL API MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 48
PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030(USD BILLION)
TABLE 49
PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030(USD BILLION)
TABLE 50
NORTH AMERICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 51
EUROPE: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 52
ASIA PACIFIC: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 53
LATIN AMERICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 54
MIDDLE EAST: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030(USD BILLION)
TABLE 55
GCC COUNTRIES: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030(USD BILLION)
TABLE 56
PARENTERAL MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 57
NORTH AMERICA: PARENTERAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 58
EUROPE: PARENTERAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 59
ASIA PACIFIC: PARENTERAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 60
LATIN AMERICA: PARENTERAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 61
MIDDLE EAST: PARENTERAL MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 62
GCC COUNTRIES: PARENTERAL MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 63
TABLET MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 64
NORTH AMERICA: TABLET MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 65
EUROPE: TABLET MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 66
ASIA PACIFIC: TABLET MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 67
LATIN AMERICA: TABLET MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 68
MIDDLE EAST: TABLET MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 69
GCC COUNTRIES: TABLET MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 70
CAPSULE MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 71
NORTH AMERICA: CAPSULE MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 72
EUROPE: CAPSULE MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 73
ASIA PACIFIC: CAPSULE MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 74
LATIN AMERICA: CAPSULE MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 75
MIDDLE EAST: CAPSULE MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 76
GCC COUNTRIES: CAPSULE MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 77
ORAL LIQUID MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 78
NORTH AMERICA: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 79
EUROPE: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 80
ASIA PACIFIC: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 81
LATIN AMERICA: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 82
MIDDLE EAST: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 83
GCC COUNTRIES: ORAL LIQUID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 84
SEMI-SOLID MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 85
NORTH AMERICA: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 86
EUROPE: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 87
ASIA PACIFIC: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 88
LATIN AMERICA: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 89
MIDDLE EAST: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 90
GCC COUNTRIES: SEMI-SOLID MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 91
OTHER FDF MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 92
NORTH AMERICA: OTHER FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 93
EUROPE: OTHER FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 94
ASIA PACIFIC: OTHER FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 95
LATIN AMERICA: OTHER FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 96
MIDDLE EAST: OTHER FDF MANUFACTURING SERVICES MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 97
GCC COUNTRIES: OTHER FDF MANUFACTURING SERVICES MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 98
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 99
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 100
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 101
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 102
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 103
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 104
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR DRUG DEVELOPMENT SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 105
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 106
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 107
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 108
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 109
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 110
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 111
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 112
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 113
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 114
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 115
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 116
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 117
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 118
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 119
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS API MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 120
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 121
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 122
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 123
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 124
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 125
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 126
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS FDF MANUFACTURING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 127
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 128
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 129
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 130
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 131
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 132
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 133
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR PACKAGING & LABELING SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 134
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 135
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 136
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 137
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 138
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 139
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL- FINISH SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 140
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR FILL-FINISH SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 141
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 142
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 143
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 144
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 145
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 146
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY REGION, 2023–2030 (USD BILLION)
TABLE 147
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SERVICES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 148
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 149
PHARMACEUTICAL MANUFACTURING SERVICES MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 150
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 151
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 152
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 153
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 154
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 155
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 156
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 157
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 158
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 159
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 160
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 161
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 162
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH-POTENCY SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 163
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR HIGH POTENCY SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 164
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY REGION, 2023–2030 (USD BILLION)
TABLE 165
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 166
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 167
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 168
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 169
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY REGION, 2023–2030 (USD BILLION)
TABLE 170
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OLIGONUCLEOTIDES AND SYNTHETIC PEPTIDES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 171
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 172
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 173
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 174
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 175
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 176
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 177
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR RADIOPHARMACEUTICAL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 178
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 179
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 180
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 181
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 182
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 183
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 184
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER SMALL MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 185
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 186
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 187
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 188
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 189
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 190
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 191
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 192
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 193
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 194
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 195
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 196
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 197
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 198
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 199
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR MONOCLONAL ANTIBODIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 200
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 201
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 202
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 203
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 204
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 205
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 206
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR CELL & GENE THERAPIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 207
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY REGION, 2023–2030 (USD BILLION)
TABLE 208
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 209
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 210
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 211
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 212
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY REGION, 2023–2030 (USD BILLION)
TABLE 213
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR ANTIBODY DRUG CONJUGATES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 214
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY REGION, 2023–2030 (USD BILLION)
TABLE 215
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 216
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 217
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 218
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 219
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY REGION, 2023–2030 (USD BILLION)
TABLE 220
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR VACCINES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 221
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY REGION, 2023–2030 (USD BILLION)
TABLE 222
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 223
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 224
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 225
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 226
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY REGION, 2023–2030 (USD BILLION)
TABLE 227
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR THERAPEUTIC PEPTIDES & PROTEINS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 228
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 229
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 230
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 231
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 232
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 233
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY REGION, 2023–2030 (USD BILLION)
TABLE 234
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER LARGE MOLECULES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 235
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 236
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 237
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 238
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 239
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 240
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 241
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 242
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIG PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 243
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 244
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 245
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 246
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 247
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 248
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 249
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL & MEDIUM-SIZED PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 250
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 251
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 252
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 253
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 254
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 255
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY REGION, 2023–2030 (USD BILLION)
TABLE 256
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR GENERIC PHARMACEUTICAL COMPANIES, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 257
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY REGION, 2023–2030 (USD BILLION)
TABLE 258
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 259
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 260
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 261
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 262
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY REGION, 2023–2030 (USD BILLION)
TABLE 263
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR OTHER END USERS, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 264
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 265
NORTH AMERICA: KEY MACROECONOMIC INDICATORS
TABLE 266
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 267
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 268
NORTH AMERICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 269
NORTH AMERICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 270
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 271
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 272
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 273
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 274
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 275
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 276
US: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 277
US: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 278
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 279
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 280
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 281
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 282
US: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 283
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 284
CANADA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 285
CANADA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 286
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 287
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 288
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 289
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 290
CANADA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 291
EUROPE: KEY MACROECONOMIC INDICATORS
TABLE 292
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 293
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 294
EUROPE: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 295
EUROPE: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 296
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 297
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 298
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 299
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 300
EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 301
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 302
GERMANY: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 303
GERMANY: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 304
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 305
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 306
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 307
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 308
GERMANY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 309
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 310
UK: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 311
UK: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 312
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 313
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 314
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 315
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 316
UK: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 317
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 318
FRANCE: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 319
FRANCE: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 320
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 321
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 322
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 323
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 324
FRANCE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 325
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 326
ITALY: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 327
ITALY: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 328
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 329
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 330
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 331
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 332
ITALY: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 333
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 334
SWITZERLAND: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 335
SWITZERLAND: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 336
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 337
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 338
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 339
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 340
SWITZERLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 341
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 342
POLAND: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 343
POLAND: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 344
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 345
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 346
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 347
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 348
POLAND: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 349
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 350
SPAIN: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 351
SPAIN: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 352
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 353
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 354
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 355
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 356
SPAIN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 357
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 358
REST OF EUROPE: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 359
REST OF EUROPE: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 360
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 361
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 362
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 363
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 364
REST OF EUROPE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 365
ASIA PACIFIC: KEY MACROECONOMIC INDICATORS
TABLE 366
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 367
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 368
ASIA PACIFIC: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 369
ASIA PACIFIC: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 370
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 371
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 372
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 373
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 374
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 375
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 376
CHINA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 377
CHINA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 378
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 379
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 380
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 381
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 382
CHINA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 383
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 384
JAPAN: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 385
JAPAN: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 386
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 387
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 388
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 389
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 390
JAPAN: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 391
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 392
SOUTH KOREA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 393
SOUTH KOREA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 394
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 395
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 396
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 397
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 398
SOUTH KOREA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 399
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 400
INDIA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 401
INDIA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 402
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 403
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 404
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 405
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 406
INDIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 407
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 408
AUSTRALIA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 409
AUSTRALIA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 410
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 411
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 412
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 413
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 414
AUSTRALIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 415
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 416
REST OF ASIA PACIFIC: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 417
REST OF ASIA PACIFIC: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 418
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 419
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 420
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 421
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 422
REST OF ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 423
LATIN AMERICA: KEY MACROECONOMIC INDICATORS
TABLE 424
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 425
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 426
LATIN AMERICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 427
LATIN AMERICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 428
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 429
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 430
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 431
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 432
LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 433
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 434
BRAZIL: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 435
BRAZIL: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 436
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 437
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 438
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 439
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 440
BRAZIL: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 441
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 442
MEXICO: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 443
MEXICO: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 444
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 445
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 446
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 447
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 448
MEXICO: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 449
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 450
REST OF LATIN AMERICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 451
REST OF LATIN AMERICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 452
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 453
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 454
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 455
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 456
REST OF LATIN AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 457
MIDDLE EAST: KEY MACROECONOMIC INDICATORS
TABLE 458
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY REGION, 2023–2030 (USD BILLION)
TABLE 459
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 460
MIDDLE EAST: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 461
MIDDLE EAST: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 462
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 463
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 464
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 465
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 466
MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 467
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY COUNTRY, 2023–2030 (USD BILLION)
TABLE 468
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 469
GCC COUNTRIES: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 470
GCC COUNTRIES: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 471
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 472
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 473
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 474
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 475
GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 476
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 477
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 478
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 479
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 480
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 481
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 482
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 483
KINGDOM OF SAUDI ARABIA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 484
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 485
UAE: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 486
UAE: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 487
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 488
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 489
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 490
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 491
UAE: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 492
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 493
REST OF GCC COUNTRIES: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 494
REST OF GCC COUNTRIES: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 495
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 496
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 497
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 498
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 499
REST OF GCC COUNTRIES: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 500
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 501
REST OF MIDDLE EAST: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 502
REST OF MIDDLE EAST: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 503
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 504
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 505
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 506
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 507
REST OF MIDDLE EAST: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION)
TABLE 508
AFRICA: KEY MACROECONOMIC INDICATORS
TABLE 509
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2023–2030 (USD BILLION)
TABLE 510
AFRICA: PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 511
AFRICA: PHARMACEUTICAL FDF MANUFACTURING SERVICES MARKET, BY TYPE, 2023–2030 (USD BILLION)
TABLE 512
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR BIOLOGICS MANUFACTURING SERVICES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 513
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2023–2030 (USD BILLION)
TABLE 514
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR SMALL MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 515
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET FOR LARGE MOLECULES, BY TYPE, 2023–2030 (USD BILLION)
TABLE 516
AFRICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2023–2030 (USD BILLION) S
TABLE 517
OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, JANUARY 2022–AUGUST 2025
TABLE 518
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: DEGREE OF COMPETITION
TABLE 519
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: REGION FOOTPRINT
TABLE 520
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: SERVICE FOOTPRINT
TABLE 521
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: MOLECULE FOOTPRINT
TABLE 522
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: DETAILED LIST OF KEY STARTUPS/SMES
TABLE 523
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
TABLE 524
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: SERVICE LAUNCHES, JANUARY 2022–JULY 2025
TABLE 525
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: DEALS, JANUARY 2022–JULY 2025
TABLE 526
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 527
THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
TABLE 528
THERMO FISHER SCIENTIFIC INC.: SERVICES OFFERED
TABLE 529
THERMO FISHER SCIENTIFIC INC.: SERVICE LAUNCHES, JANUARY 2022–JULY 2025
TABLE 530
THERMO FISHER SCIENTIFIC INC.: DEALS, JANUARY 2022–JULY 2025
TABLE 531
LONZA: COMPANY OVERVIEW
TABLE 532
LONZA: SERVICES OFFERED
TABLE 533
LONZA: DEALS, JANUARY 2022–JULY 2025
TABLE 534
LONZA: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 535
CATALENT, INC.: COMPANY OVERVIEW
TABLE 536
CATALENT, INC.: SERVICES OFFERED
TABLE 537
CATALENT, INC.: SERVICE LAUNCHES, JANUARY 2022–JULY 2025
TABLE 538
CATALENT, INC.: DEALS, JANUARY 2022–JULY 2025
TABLE 539
CATALENT, INC.: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 540
WUXI APPTEC: COMPANY OVERVIEW
TABLE 541
WUXI APPTEC: SERVICES OFFERED
TABLE 542
WUXI APPTEC: DEALS, JANUARY 2022–JULY 2025
TABLE 543
WUXI APPTEC: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 544
WUXI BIOLOGICS: COMPANY OVERVIEW
TABLE 545
WUXI BIOLOGICS: SERVICES OFFERED
TABLE 546
WUXI BIOLOGICS: SERVICE LAUNCHES, JANUARY 2022–JULY 2025
TABLE 547
WUXI BIOLOGICS: DEALS, JANUARY 2022–JULY 2025
TABLE 548
WUXI BIOLOGICS: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 549
WUXI BIOLOGICS: OTHER DEVELOPMENTS, JANUARY 2022–JULY 2025
TABLE 550
SAMSUNG BIOLOGICS: COMPANY OVERVIEW
TABLE 551
SAMSUNG BIOLOGICS: SERVICES OFFERED
TABLE 552
SAMSUNG BIOLOGICS: SERVICE LAUNCHES, JANUARY 2022–JUNE 2025
TABLE 553
SAMSUNG BIOLOGICS: DEALS, JANUARY 2022–JULY 2025
TABLE 554
SAMSUNG BIOLOGICS: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 555
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: COMPANY OVERVIEW
TABLE 556
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: SERVICES OFFERED
TABLE 557
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: DEALS, JANUARY 2022–JULY 2025
TABLE 558
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 559
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: OTHER DEVELOPMENTS, JANUARY 2022–JULY 2025
TABLE 560
EVONIK: COMPANY OVERVIEW
TABLE 561
EVONIK: SERVICES OFFERED
TABLE 562
EVONIK: DEALS, JANUARY 2022–JULY 2025
TABLE 563
EVONIK: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 564
FUJIFILM HOLDINGS CORPORATION: COMPANY OVERVIEW
TABLE 565
FUJIFILM HOLDINGS CORPORATION: SERVICES OFFERED
TABLE 566
FUJIFILM HOLDINGS CORPORATION: DEALS, JANUARY 2022–JULY 2025
TABLE 567
FUJIFILM HOLDINGS CORPORATION: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 568
ABBVIE INC.: COMPANY OVERVIEW
TABLE 569
ABBVIE INC.: SERVICES OFFERED
TABLE 570
ABBVIE INC.: DEALS, JANUARY 2022–JULY 2025
TABLE 571
ABBVIE INC.: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 572
SIEGFRIED HOLDING AG: COMPANY OVERVIEW
TABLE 573
SIEGFRIED HOLDING AG: SERVICES OFFERED
TABLE 574
SIEGFRIED HOLDING AG: DEALS, JANUARY 2022–JULY 2025
TABLE 575
SIEGFRIED HOLDING AG: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 576
MERCK KGAA: COMPANY OVERVIEW
TABLE 577
MERCK KGAA: SERVICES OFFERED
TABLE 578
MERCK KGAA: DEALS, JANUARY 2022–JULY 2025
TABLE 579
MERCK KGAA: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 580
ALMAC GROUP: COMPANY OVERVIEW
TABLE 581
ALMAC GROUP: SERVICES OFFERED
TABLE 582
ALMAC GROUP: DEALS, JANUARY 2022–JULY 2025
TABLE 583
ALMAC GROUP: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 584
CHARLES RIVER LABORATORIES: COMPANY OVERVIEW
TABLE 585
CHARLES RIVER LABORATORIES: SERVICES OFFERED
TABLE 586
CHARLES RIVER LABORATORIES: DEALS, JANUARY 2022–JULY 2025
TABLE 587
CHARLES RIVER LABORATORIES: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 588
ASYMCHEM INC.: COMPANY OVERVIEW
TABLE 589
ASYMCHEM INC.: SERVICES OFFERED
TABLE 590
ASYMCHEM INC.: DEALS, JANUARY 2022–JULY 2025
TABLE 591
ASYMCHEM INC.: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 592
VETTER: COMPANY OVERVIEW
TABLE 593
VETTER: SERVICES OFFERED
TABLE 594
VETTER: SERVICE LAUNCHES, JANUARY 2022–JULY 2025
TABLE 595
VETTER: DEALS, JANUARY 2022–JULY 2025
TABLE 596
VETTER: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 597
ALCAMI CORPORATION: COMPANY OVERVIEW
TABLE 598
ALCAMI CORPORATION: SERVICES OFFERED
TABLE 599
ALCAMI CORPORATION: DEALS, JANUARY 2022–JULY 2025
TABLE 600
ALCAMI CORPORATION: EXPANSIONS, JANUARY 2022–JULY 2025
TABLE 601
EUROFINS SCIENTIFIC: COMPANY OVERVIEW
TABLE 602
EUROFINS SCIENTIFIC: SERVICES OFFERED
TABLE 603
EUROFINS SCIENTIFIC: EXPANSIONS, JANUARY 2022–JUNE 2025
TABLE 604
PIRAMAL PHARMA SOLUTIONS: COMPANY OVERVIEW
TABLE 605
SYNGENE INTERNATIONAL LIMITED: COMPANY OVERVIEW
TABLE 606
CAMBREX CORPORATION: COMPANY OVERVIEW
TABLE 607
JUBILANT PHARMANOVA LIMITED: COMPANY OVERVIEW
TABLE 608
YUHAN CORPORATION: COMPANY OVERVIEW
TABLE 609
PIERRE FABRE LABORATORIES: COMPANY OVERVIEW
TABLE 610
PFIZER CENTREONE: COMPANY OVERVIEW
TABLE 611
DELPHARM: COMPANY OVERVIEW
TABLE 612
FRONTAGE LABS: COMPANY OVERVIEW
TABLE 613
SHARP SERVICES, LLC: COMPANY OVERVIEW
TABLE 614
GRAND RIVER ASEPTIC MANUFACTURING: COMPANY OVERVIEW
TABLE 615
RECIPHARM AB: COMPANY OVERVIEW
TABLE 616
PCI PHARMA SERVICES: COMPANY OVERVIEW
TABLE 617
CURIA GLOBAL, INC.: COMPANY OVERVIEW
TABLE 618
SIMTRA BIOPHARMA SOLUTIONS: COMPANY OVERVIEW
TABLE 619
PORTON: COMPANY OVERVIEW
TABLE 620
MABPLEX INTERNATIONAL CO. LTD.: COMPANY OVERVIEW
TABLE 621
CELLARES: COMPANY OVERVIEW
FIGURE 1
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SEGMENTATION AND REGIONAL SCOPE
FIGURE 2
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: YEARS CONSIDERED
FIGURE 3
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: RESEARCH DESIGN
FIGURE 4
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: KEY DATA FROM SECONDARY SOURCES
FIGURE 5
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: BREAKDOWN OF PRIMARY INTERVIEWS
FIGURE 6
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2024
FIGURE 7
COMPANY REVENUE ANALYSIS-BASED ESTIMATION: BOTTOM-UP APPROACH, 2024
FIGURE 8
REVENUE SHARE ANALYSIS OF THERMO FISHER SCIENTIFIC INC., 2024
FIGURE 9
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SIZE VALIDATION FROM PRIMARY SOURCES
FIGURE 10
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: TOP-DOWN APPROACH
FIGURE 11
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: CAGR PROJECTIONS
FIGURE 12
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: DATA TRIANGULATION
FIGURE 13
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY SERVICE, 2025 VS. 2030 (USD BILLION)
FIGURE 14
PHARMACEUTICAL MANUFACTURING SERVICES MARKET, BY TYPE, 2025 VS. 2030 (USD BILLION)
FIGURE 15
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY MOLECULE, 2025 VS. 2030 (USD BILLION)
FIGURE 16
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY END USER, 2025 VS. 2030 (USD BILLION)
FIGURE 17
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET, BY REGION, 2025–2030
FIGURE 18
RISING INVESTMENTS IN PHARMACEUTICAL R&D TO DRIVE MARKET
FIGURE 19
PHARMACEUTICAL MANUFACTURING SERVICES AND US TO LEAD NORTH AMERICAN MARKET IN 2025
FIGURE 20
SMALL MOLECULES SEGMENT TO DOMINATE MARKET DURING FORECAST PERIOD
FIGURE 21
BIG PHARMACEUTICAL COMPANIES SEGMENT TO ACCOUNT FOR LARGEST MARKET SHARE DURING FORECAST PERIOD
FIGURE 22
CHINA TO HAVE LARGEST MARKET DURING FORECAST PERIOD
FIGURE 23
EMERGING MARKETS TO REGISTER HIGHER GROWTH DURING FORECAST PERIOD
FIGURE 24
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
FIGURE 25
NEW REVENUE POCKETS FOR PLAYERS IN PHARMACEUTICAL CONTRACT MANUFACTURING MARKET
FIGURE 26
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: VALUE CHAIN ANALYSIS
FIGURE 27
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: SUPPLY CHAIN ANALYSIS
FIGURE 28
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: ECOSYSTEM ANALYSIS
FIGURE 29
INVESTMENT AND FUNDING SCENARIO, 2023–2024
FIGURE 30
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: PATENT ANALYSIS, JANUARY 2014–DECEMBER 2024
FIGURE 31
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: PORTER’S FIVE FORCES ANALYSIS
FIGURE 32
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY SERVICE
FIGURE 33
KEY BUYING CRITERIA FOR END USERS
FIGURE 34
ABBREVIATED NEW DRUG APPLICATION (ANDA) APPROVALS, 2020–2025
FIGURE 35
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: AI USE CASES
FIGURE 36
NORTH AMERICA: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SNAPSHOT
FIGURE 37
ASIA PACIFIC: PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SNAPSHOT
FIGURE 39
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
FIGURE 40
EV/EBITDA OF KEY VENDORS
FIGURE 41
YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
FIGURE 42
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: BRAND/SERVICE COMPARISON
FIGURE 43
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
FIGURE 44
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: COMPANY FOOTPRINT
FIGURE 45
PHARMACEUTICAL CONTRACT MANUFACTURING MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
FIGURE 46
THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2024)
FIGURE 47
LONZA: COMPANY SNAPSHOT (2024)
FIGURE 48
CATALENT, INC.: COMPANY SNAPSHOT (2023)
FIGURE 49
WUXI APPTEC: COMPANY SNAPSHOT (2024)
FIGURE 50
WUXI BIOLOGICS: COMPANY SNAPSHOT (2024)
FIGURE 51
SAMSUNG BIOLOGICS: COMPANY SNAPSHOT (2024)
FIGURE 52
BOEHRINGER INGELHEIM INTERNATIONAL GMBH: COMPANY SNAPSHOT (2024)
FIGURE 53
EVONIK: COMPANY SNAPSHOT (2024)
FIGURE 54
FUJIFILM HOLDINGS CORPORATION: COMPANY SNAPSHOT (2024)
FIGURE 55
ABBVIE INC.: COMPANY SNAPSHOT (2024)
FIGURE 56
SIEGFRIED HOLDING AG: COMPANY SNAPSHOT (2024)
FIGURE 57
MERCK KGAA: COMPANY SNAPSHOT (2024)
FIGURE 58
ALMAC GROUP: COMPANY SNAPSHOT (2024)
FIGURE 59
CHARLES RIVER LABORATORIES: COMPANY SNAPSHOT (2024)
FIGURE 60
ASYMCHEM INC.: COMPANY SNAPSHOT (2024)
FIGURE 61
VETTER: COMPANY SNAPSHOT (2024)
FIGURE 62
EUROFINS SCIENTIFIC: COMPANY SNAPSHOT (2024)





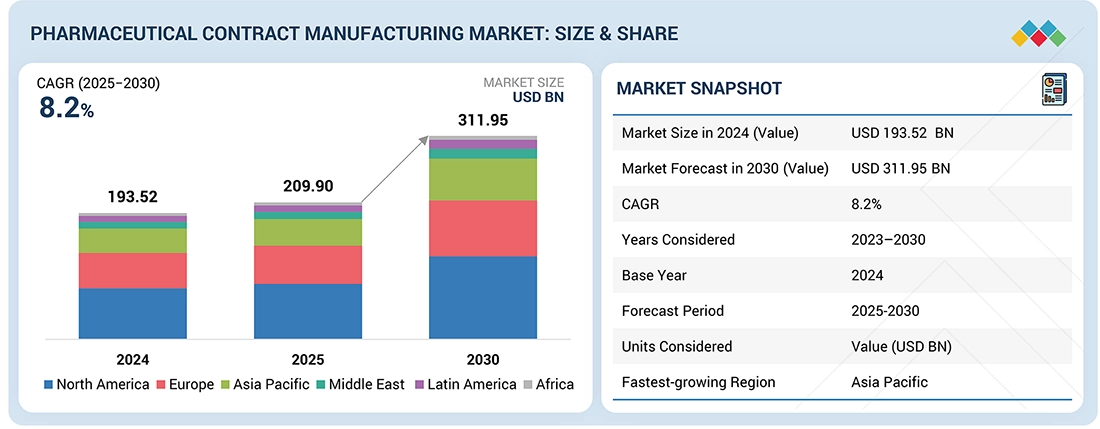
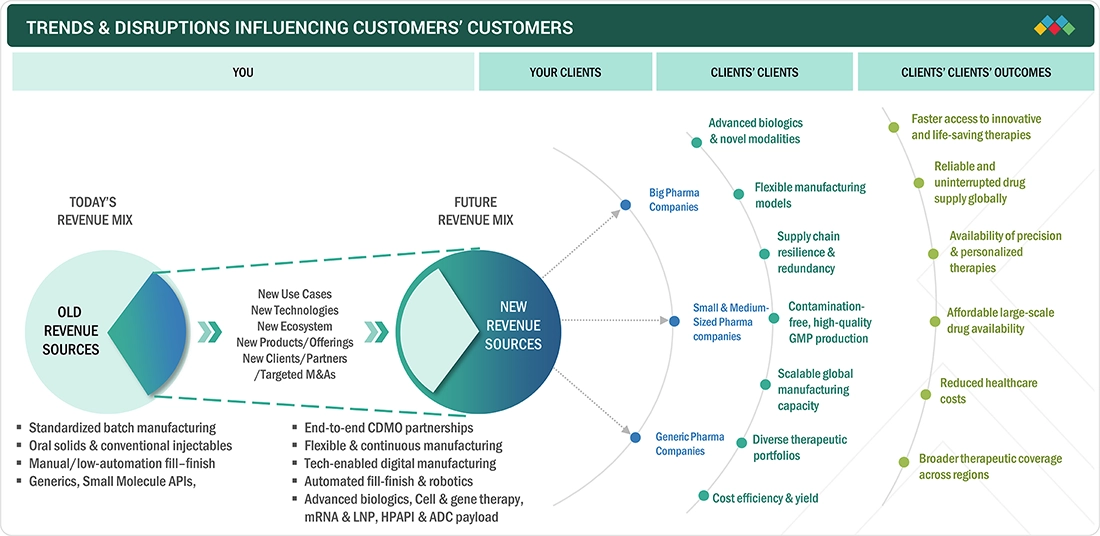





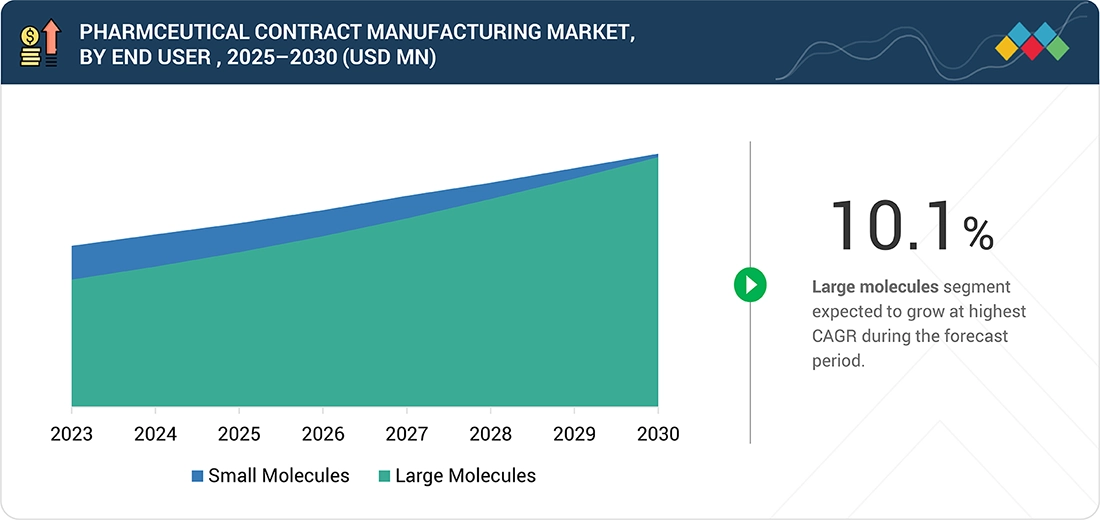

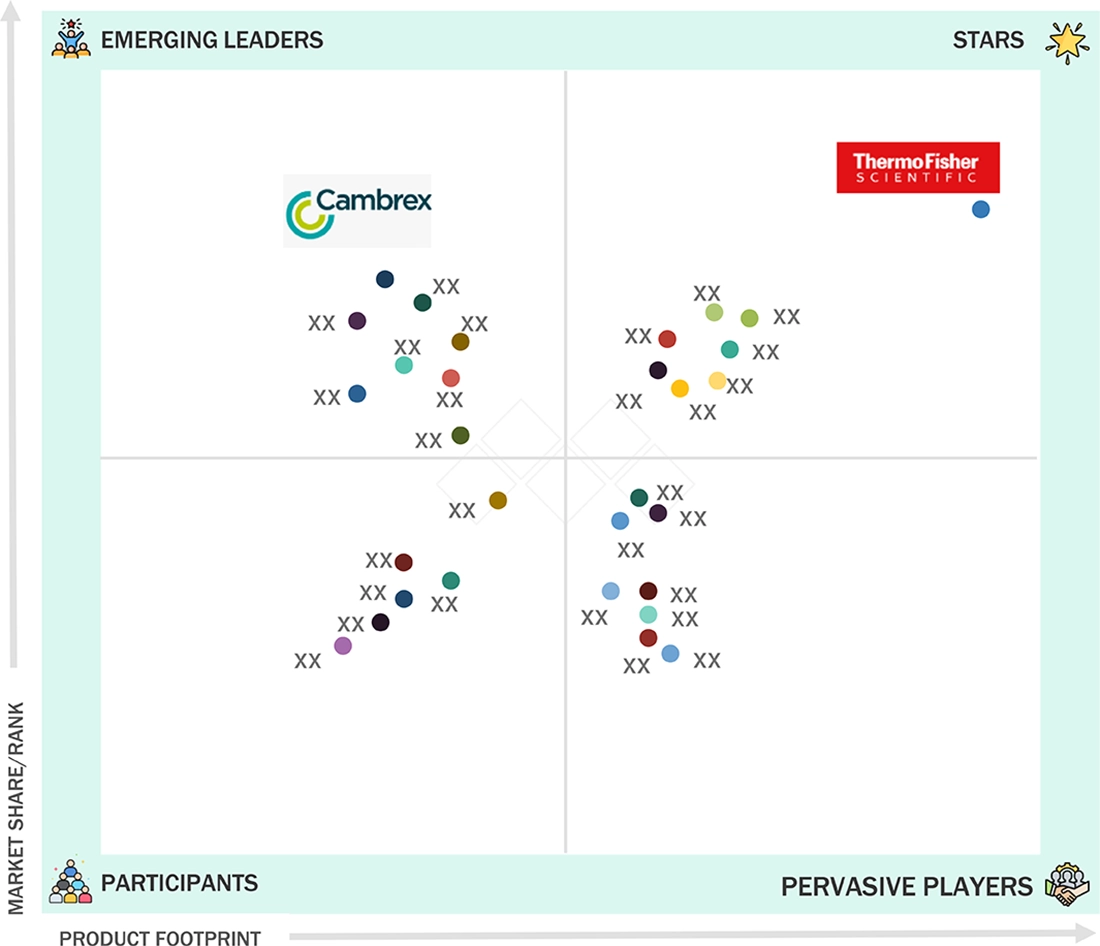
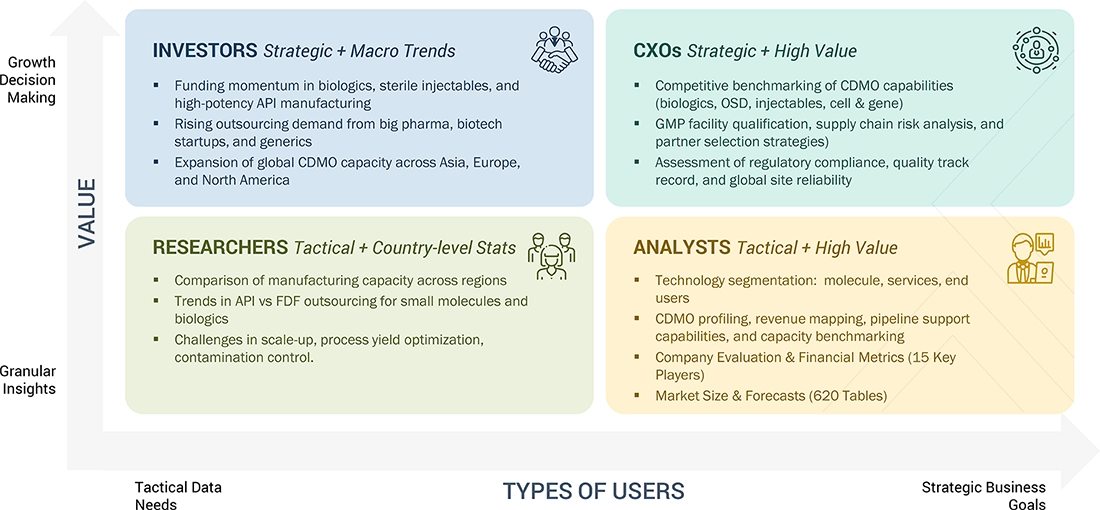

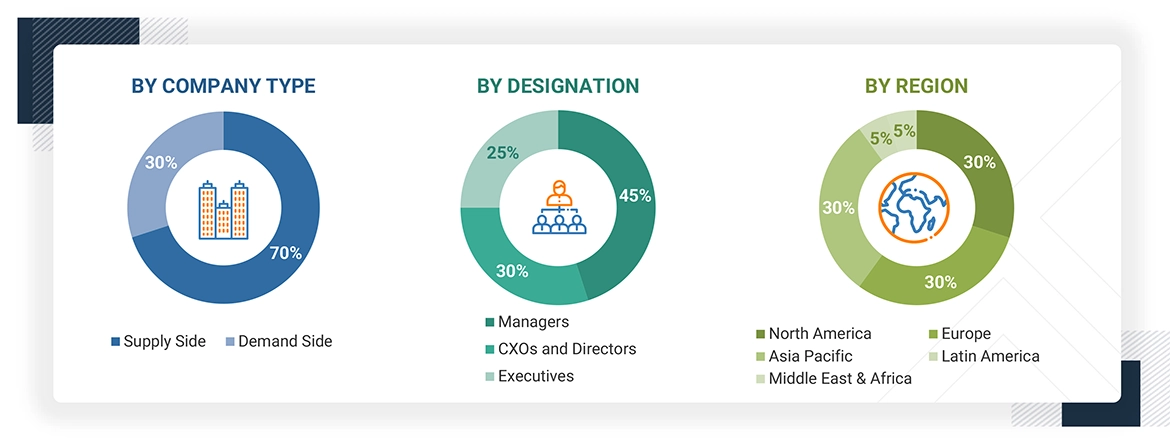















Kahlill
Feb, 2023
What will be the key challenges for business in the pharmaceutical contract manufacturing market in the near future?.
MnM Analyst
Jul, 2023
Key players in pharmaceutical contract manufacturing industry for North America region include:
- Pfizer
- Merck & Co., Inc.
- Boehringer Ingelheim
- AbbVie Inc.
- Roche
- Novartis
- GlaxoSmithKline
- Sanofi
- AstraZeneca
- Eli Lilly and Company among others
.James
Jul, 2023
Who are the top key players in the pharmaceutical contract manufacturing market in North America?.