Cancer Diagnostics Market by Product (Consumables (Antibodies, Probes), Instruments (Pathology Instruments, Imaging Instruments, Biopsy), Technology (IVD Testing), Application (Breast Cancer, Lung Cancer), End User (Hospitals) & Region - Global Forecast to 2026
Market Growth Outlook Summary
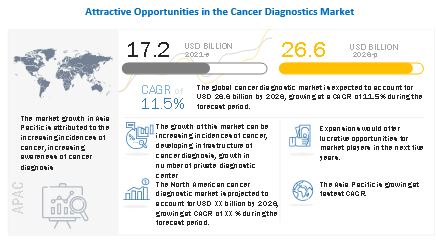
The global cancer diagnostics market growth forecasted to transform from $17.2 billion in 2021 to $26.6 billion by 2026, driven by a CAGR of 11.5%. This growth is driven by the increasing incidence of cancer and the rise in private diagnostic centers. Opportunities are enhanced by government and healthcare provider recommendations for early cancer screening. However, the market faces challenges with the adoption of refurbished diagnostic imaging systems due to cost constraints. The consumables segment is expected to grow the fastest, while IVD testing technology holds the largest market share. Hospitals are the largest end-users, and North America dominates the regional market. Key players include GE Healthcare, Roche Diagnostics, and Becton, Dickinson and Company. Recent developments include partnerships for improving patient outcomes and expanding access to advanced diagnostic technologies.
To know about the assumptions considered for the study, download the pdf brochure
Cancer Diagnostics Market Dynamics
Driver: Growth in the number of private diagnostic centers
The number of private diagnostic centers is increasing across the globe as there is a rising demand for diagnostic imaging procedures and a growing burden on public hospitals due to the limited number of imaging modalities at their disposal. In January 2021, FUJIFILM Corporation opened NURA, a medical screening center focusing on cancer screening in Bangalore, India. This medical screening center is operated by FUJIFILM DKH LLP (FUJIFILM DKH) and Dr. Kutty’s Healthcare (DKH). FUJIFILM DKH LLP (FUJIFILM DKH) is a joint venture of FUJIFILM and Dr. Kutty’s Healthcare (DKH), which runs hospitals and screening centers in India and the Middle East.
Opportunity: Recommendations for cancer screening
In an attempt to detect cancer in the early stages and bring down the mortality rate, governments in developed countries and primary care doctors are recommending cancer screening tests for patients. The US Preventive Services Task Force (USPSTF) recommends screening for colorectal cancer starting at 50 years of age to 75 years of age in the US as a means of preventing disease incidence and ensuring early-stage treatment. Canada has also implemented guidelines for biennial colorectal cancer screening for people aged 50 to 74 years.
Challenge: Increasing adoption of refurbished diagnostic imaging systems
Many hospitals in developing countries cannot invest in diagnostic imaging equipment due to their higher cost, poor reimbursement rates, and budgetary constraints. However, due to the high demand for diagnostic imaging procedures in these nations, hospitals that cannot afford to invest in new imaging systems prefer to opt for refurbished ones. Refurbished systems are priced lower than new systems and are approximately in the range of 40% to 60% of the original price of the equipment.
Owing to this, many market leaders are now promoting refurbished devices through various programs. For instance, Siemens’ Medical Proven Excellence Program, GE Healthcare’s Gold Seal Program, and Philips’ Diamond Select Program are some noteworthy global refurbishing programs that promote the utilization of refurbished diagnostic imaging systems.
The consumables segment of cancer diagnostics industry, is expected to grow at the highest CAGR during the forecast period
Based on the product, the market is segmented into consumables and instruments. The consumables segment is projected to witness the highest growth during the forecast period. The repeated purchase and high consumption, and the high prevalence of diseases the major factors supporting the growth of this segment.
The technology segment accounted for the largest share of the cancer diagnostics industry
By technology, the market is segmented IVD testing, imaging based and biopsy technique. The IVD testing segment accounted for the largest market share. The large share of this segment can be attributed to increasing incidence of cancer.
Hospitals are the largest share of the cancer diagnostics industry
Based on end-users, the market is segmented into hospitals and diagnostic laboratories. The hospitals segment accounted for the largest share of the market. The increasing number of patients visiting hospitals, rising number of in-house diagnostic procedures performed in hospitals, and growing awareness regarding early diagnosis is are the major driving factor for this market.
North America accounted for the largest share of the cancer diagnostics industry
Based on the region, the market is segmented into North America, Europe, Asia Pacific, and the Rest of the World (RoW). North America accounted for the largest share of the market. The large share of North America can be attributed to factors such as increasing incidence of cancer and technological advancement.
Some of the major players operating in this market are GE Healthcare (US), Roche Diagnostics (Switzerland), and Becton, Dickinson and Company (US). In 2020, GE Healthcare held the leading position in the market. The company has a strong geographic presence across the US, Asia, Europe, Middle East and Africa, & the Americas. Moreover, the company’s strong brand recognition and comprehensive product portfolio in the cancer diagnostics market is its key strength. BD held the second position in the market in 2020.
Scope of the Cancer Diagnostics Industry
|
Report Metric |
Details |
|
Market Revenue Size in 2021 |
$17.2 billion |
|
Projected Revenue Size by 2026 |
$26.6 billion |
|
Industry Growth Rate |
Poised to grow at a CAGR of 11.5% |
|
Market Drivers |
Growth in the number of private diagnostic centers |
|
Market Opportunities |
Recommendations for cancer screening |
This research report categorizes the cancer diagnostics market to forecast revenue and analyze trends in each of the following submarkets:
By Product
-
Consumables
- Antibodies
- Kits & Reagents
- Probes
- Other Consumables
-
Instruments
-
Pathology-based Instruments
- Slide Staining Systems
- Tissue Processing Systems
- Cell Processors
- PCR Instruments
- NGS Instruments
- Microarrays
- Other Pathology-based Instruments
-
Imaging Instruments
- CT Systems
- Ultrasound Systems
- MRI Systems
- Mammography Systems
- Nuclear Imaging Systems
-
Pathology-based Instruments
- Biopsy Instruments
By Technology
-
IVD Testing
- Polymerase Chain Reaction (PCR)
- In Situ Hybridization (ISH)
- Immunohistochemistry (IHC)
- Next-generation Sequencing (NGS)
- Microarrays
- Flow Cytometry
- Immunoassays
- Other IVD Testing Technologies
-
Imaging
- Magnetic Resonance Imaging (MRI)
- Computed Tomography (CT)
- Positron Emission Tomography (PET)
- Mammography
- Ultrasound
- Biopsy Technique
By Application
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Melanoma
- Other Cancers
By End User
- Hospitals
- Diagnostic Laboratories
By Region
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Spain
- Italy
- RoE
-
Asia Pacific
- China
- India
- Japan
- RoAPAC
-
Rest of the World
- Latin America
- Middle East and Africa
Recent Developments of Cancer Diagnostics Industry:
- In 2020, GE Healthcate entered into partnership with GenesisCare to improve patient outcomes for the two biggest health burdens globally, cancer and heart disease. GE Healthcare will provide CT, MRI, PET/CT, SPECT, digital mammography, and ultrasound equipment to GenesisCare’s 440+ cancer and cardiovascular disease treatment centers across Australia, the US, the UK, and Spain
- In 2020, Roche Diagnostics entered into partnership with Illumina to provide broad access to clinical oncology next-generation sequencing
Frequently Asked Questions (FAQ):
What is the projected market revenue value of the global cancer diagnostics market?
The global cancer diagnostics market boasts a total revenue value of $26.6 billion by 2026.
What is the estimated growth rate (CAGR) of the global cancer diagnostics market?
The global cancer diagnostics market has an estimated compound annual growth rate (CAGR) of 11.5% and a revenue size in the region of $17.2 billion in 2021.
To speak to our analyst for a discussion on the above findings, click Speak to Analyst

TABLE OF CONTENTS
1 INTRODUCTION (Page No. - 27)
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.2.1 INCLUSIONS AND EXCLUSIONS OF THE STUDY
1.3 MARKET SCOPE
1.3.1 MARKET SEGMENTATION
1.3.2 YEARS CONSIDERED FOR THE STUDY
1.4 CURRENCY
1.5 LIMITATIONS
1.6 MARKET STAKEHOLDERS
1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY (Page No. - 31)
2.1 RESEARCH DATA
2.1.1 SECONDARY DATA
2.1.1.1 Secondary sources
2.1.2 PRIMARY DATA
2.1.2.1 Key data from primary sources
2.1.2.2 Key industry insights
2.2 CANCER DIAGNOSTICS MARKET SIZE ESTIMATION
2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
2.4 MARKET SHARE ANALYSIS
2.5 ASSUMPTIONS FOR THE STUDY
2.6 COVID-19 HEALTH ASSESSMENT
2.7 COVID-19 ECONOMIC ASSESSMENT
2.8 ASSESSMENT OF THE IMPACT OF COVID-19 ON THE ECONOMIC SCENARIO
3 EXECUTIVE SUMMARY (Page No. - 44)
4 PREMIUM INSIGHTS (Page No. - 48)
4.1 CANCER DIAGNOSTICS MARKET OVERVIEW
4.2 MARKET, BY PRODUCT
4.3 NORTH AMERICA: MARKET, BY PRODUCT AND COUNTRY, 2020
4.4 GEOGRAPHICAL SNAPSHOT OF THE MARKET
5 MARKET OVERVIEW (Page No. - 51)
5.1 INTRODUCTION
5.2 CANCER DIAGNOSTICS MARKET DYNAMICS
5.2.1 DRIVERS
5.2.1.1 Increasing incidence of cancer
5.2.1.2 Growth in the number of private diagnostic centers
5.2.1.3 Availability of reimbursement
5.2.2 RESTRAINTS
5.2.2.1 High capital investments and low cost-benefit ratio for biomarkers
5.2.2.2 High cost of diagnostic imaging systems
5.2.2.3 Product recalls and failure
5.2.3 OPPORTUNITIES
5.2.3.1 Growing focus on personalized medicine
5.2.3.2 Companion diagnostics
5.2.3.3 Recommendations for cancer screening
5.2.3.4 Emerging countries
5.2.4 CHALLENGES
5.2.4.1 Increasing adoption of refurbished diagnostic imaging systems
5.2.4.2 Lack of skilled professionals
5.2.4.3 Inadequate infrastructure and low awareness in middle- and low-income countries
5.2.5 TRENDS
5.2.5.1 Rental agreements
5.3 COVID-19 IMPACT ANALYSIS
5.4 VALUE CHAIN ANALYSIS
5.5 SUPPLY CHAIN ANALYSIS
5.6 PORTER’S FIVE FORCES ANALYSIS
5.6.1 THREAT FROM NEW ENTRANTS
5.6.2 INTENSITY OF COMPETITIVE RIVALRY
5.6.3 BARGAINING POWER OF BUYERS
5.6.4 BARGAINING POWER OF SUPPLIERS
5.6.5 THREAT FROM SUBSTITUTES
5.7 TECHNOLOGY ANALYSIS
5.8 REGULATORY LANDSCAPE
5.9 PATENT ANALYSIS
5.10 TRADE ANALYSIS
5.10.1 TRADE ANALYSIS FOR MAMMOGRAPHY SYSTEMS
5.10.2 TRADE ANALYSIS FOR MAGNETIC RESONANCE IMAGING (MRI) SYSTEMS
5.11 PRICING ANALYSIS
5.12 ECOSYSTEM ANALYSIS: MARKET
5.12.1 ROLE IN ECOSYSTEM
5.13 YCC SHIFT
6 CANCER DIAGNOSTICS MARKET, BY PRODUCT (Page No. - 69)
6.1 INTRODUCTION
6.2 CONSUMABLES
6.2.1 ANTIBODIES
6.2.1.1 Antibodies accounted for the largest share of the cancer diagnostic consumables market in 2020
6.2.2 KITS & REAGENTS
6.2.2.1 Use of kits is increasing among end users owing to their ease of use
6.2.3 PROBES
6.2.3.1 Abbott and Agilent Technologies are some of the leading players operating in this market segment
6.2.4 OTHER CONSUMABLES
6.3 INSTRUMENTS
6.3.1 PATHOLOGY-BASED INSTRUMENTS
6.3.1.1 Slide staining systems
6.3.1.1.1 Slide staining systems accounted for the largest share of the pathology-based instruments market
6.3.1.2 Tissue processing systems
6.3.1.2.1 Tissue processing systems are used for the automatic processing of tissue samples
6.3.1.3 Cell processors
6.3.1.3.1 Cell processors are used to remove unwanted matter from cytology samples
6.3.1.4 PCR instruments
6.3.1.4.1 Cost-effectiveness of PCR is driving the growth of this market segment
6.3.1.5 NGS instruments
6.3.1.5.1 NGS is an emerging technology for cancer diagnostics
6.3.1.6 Microarrays
6.3.1.6.1 DNA microarrays are useful for the determination of primary sites in metastatic carcinomas
6.3.1.7 Other pathology-based instruments
6.3.2 IMAGING INSTRUMENTS
6.3.2.1 CT systems
6.3.2.1.1 Technological advancements in CT to drive the growth of this market segment
6.3.2.2 Ultrasound systems
6.3.2.2.1 Increasing incidence of cancer to drive the growth of this market segment
6.3.2.3 MRI systems
6.3.2.3.1 Use of MRI in cancer diagnostics has increased over the years
6.3.2.4 Mammography systems
6.3.2.4.1 Increasing incidence of breast cancer to drive the growth of this market segment
6.3.2.5 Nuclear imaging systems
6.3.2.5.1 Technological advancements in nuclear imaging systems to drive the growth of this market segment
6.3.3 BIOPSY INSTRUMENTS
6.3.3.1 Increasing prevalence of cancer to drive the growth of this market segment
7 CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY (Page No. - 85)
7.1 INTRODUCTION
7.2 IVD TESTING
7.2.1 POLYMERASE CHAIN REACTION
7.2.1.1 Physicians prefer PCR-based diagnostic tests as they provide simple representations of genomic data
7.2.2 IN SITU HYBRIDIZATION
7.2.2.1 ISH is used for the identification of specific mRNA species within sample cells
7.2.3 IMMUNOHISTOCHEMISTRY
7.2.3.1 Increasing use of IHC tests for cancer diagnostics to boost the market growth
7.2.4 NEXT-GENERATION SEQUENCING
7.2.4.1 NGS dominated the IVD testing market in 2020
7.2.5 IMMUNOASSAY
7.2.5.1 Immunoassay segment accounted for the second-largest share of the market for IVD testing
7.2.6 MICROARRAY
7.2.6.1 Cost-effectiveness of the microarray technology to support market growth
7.2.7 FLOW CYTOMETRY
7.2.7.1 Flow cytometry tools help to detect the number of chromosomes to determine the malignancy of a tumor
7.2.8 OTHER IVD TECHNOLOGIES
7.3 IMAGING TECHNOLOGIES
7.3.1 MAGNETIC RESONANCE IMAGING
7.3.1.1 Technological advancements to boost the growth of the MRI market
7.3.2 COMPUTED TOMOGRAPHY
7.3.2.1 CT is a widely used technique in cancer diagnostics
7.3.3 NUCLEAR IMAGING
7.3.3.1 PET is a widely used nuclear imaging technique in cancer diagnostics
7.3.4 MAMMOGRAPHY
7.3.4.1 Increasing government initiativeS for breast cancer screening to drive the growth of this market segment
7.3.5 ULTRASOUND
7.3.5.1 Adoption of POC ultrasound for diagnostic purposeS to suppoRt market growth
7.4 BIOPSIES
8 CANCER DIAGNOSTICS MARKET, BY APPLICATION (Page No. - 102)
8.1 INTRODUCTION
8.2 BREAST CANCER
8.2.1 BREAST CANCER SEGMENT TO ACCOUNT FOR THE LARGEST SHARE DURING THE FORECAST PERIOD
8.3 LUNG CANCER
8.3.1 LUNG CANCER SEGMENT ACCOUNTED FOR THE SECOND-LARGEST SHARE IN 2020
8.4 COLORECTAL CANCER
8.4.1 INCREASING INCIDENCE OF COLORECTAL CANCER TO DRIVE MARKET GROWTH
8.5 MELANOMA
8.5.1 INCREASING INCIDENCE OF MELANOMA TO DRIVE MARKET GROWTH
8.6 OTHER CANCERS
9 CANCER DIAGNOSTICS MARKET, BY END USER (Page No. - 109)
9.1 INTRODUCTION
9.2 HOSPITALS
9.2.1 HOSPITALS ARE THE LARGEST END USERS OF CANCER DIAGNOSTIC PRODUCTS
9.3 DIAGNOSTIC LABORATORIES
9.3.1 INCREASING INCIDENCE OF CANCER TO DRIVE THE GROWTH OF THIS SEGMENT
10 CANCER DIAGNOSTICS MARKET, BY REGION (Page No. - 112)
10.1 INTRODUCTION
10.2 NORTH AMERICA
10.2.1 MARKET, BY REGION
10.2.1.1 US is expected to be dominant the North American market
10.2.2 NORTH AMERICA
10.2.2.1 High prevalence of cancer in Canada is expected to support market growth
10.3 EUROPE
10.3.1 CANADA
10.3.1.1 Availability of reimbursements for colorectal cancer screening to drive market growth
10.3.2 EUROPE
10.3.2.1 Government initiatives to drive market growth in the UK
10.3.3 Availability of reimbursements for colorectal cancer screening to drive market growth
10.3.3.1 Increasing government funding for cancer diagnosis and prevention to support the market growth
10.3.4 Government initiatives to drive market growth in the UK
10.3.4.1 High incidence of cancer to support the market growth in Italy
10.3.5 Increasing government funding for cancer diagnosis and prevention to support the market growth
10.3.5.1 High incidence of chronic diseases to drive the market growth in Spain
10.3.6 High incidence of cancer to support the market growth in Italy
10.4 ASIA PACIFIC
10.4.1 High incidence of chronic diseases to drive the market growth in Spain
10.4.1.1 Increasing healthcare expenditure to drive market growth in China
10.4.2 ASIA PACIFIC
10.4.2.1 Advanced healthcare infrastructure to support market growth in Japan
10.4.3 Increasing healthcare expenditure to drive market growth in China
10.4.3.1 Expanding healthcare sector in the country to drive market growth
10.4.4 Advanced healthcare infrastructure to support market growth in Japan
10.5 REST OF THE WORLD
10.5.1 Expanding healthcare sector in the country to drive market growth
10.5.1.1 Increasing number of cancer screening programs to support market growth
10.5.2 REST OF THE WORLD
10.5.2.1 Increasing incidence of cancer in the Middle East & Africa to drive market growth
11 COMPETITIVE LANDSCAPE (Page No. - 194)
11.1 OVERVIEW
11.2 CANCER DIAGNOSTICS MARKET SHARE ANALYSIS
11.3 COMPANY EVALUATION MATRIX
11.3.1 OVERVIEW
11.3.2 MARKET SHARE ANALYSIS
11.3.3 COMPANY EVALUATION MATRIX
11.3.4 STARS
11.4 COMPETITIVE LEADERSHIP MAPPING (SMES/ START-UPS)
11.4.1 PERVASIVE PLAYERS
11.4.2 PARTICIPANTS
11.4.3 COMPETITIVE LEADERSHIP MAPPING (SMES/ START-UPS)
11.4.4 PROGRESSIVE COMPANIES
11.5 COMPETITIVE SCENARIO
11.5.1 RESPONSIVE COMPANIES
11.5.2 DYNAMIC COMPANIES
11.5.3 COMPETITIVE SCENARIO
11.5.4 PRODUCT LAUNCHES & APPROVALS
12 COMPANY PROFILES (Page No. - 208)
12.1 KEY PLAYERS
12.1.1 COMPANY FOOTPRINT
12.1.1.1 Business overview
12.1.1.2 Products offered
12.1.1.3 Recent developments
12.1.1.4 SWOT analysis
12.1.1.5 Right to win
12.1.2 Recent developments
12.1.2.1 Business overview
12.1.2.2 Products offered
12.1.2.3 SWOT analysis
12.1.2.4 Right to win
12.1.3 Products offered
12.1.3.1 Business overview
12.1.3.2 Products offered
12.1.3.3 Recent developments
12.1.3.4 SWOT analysis
12.1.3.5 Right to win
12.1.4 Recent developments
12.1.4.1 Business overview
12.1.4.2 Products offered
12.1.4.3 Recent developments
12.1.4.4 SWOT analysis
12.1.4.5 Right to win
12.1.5 Recent developments
12.1.5.1 Business overview
12.1.5.2 Products offered
12.1.5.3 Recent developments
12.1.5.4 SWOT analysis
12.1.5.5 Right to win
12.1.6 Recent developments
12.1.6.1 Business overview
12.1.6.2 Products offered
12.1.7 ABBOTT LABORATORIES
12.1.7.1 Business overview
12.1.7.2 Products offered
12.1.7.3 Recent developments
12.1.8 Business overview
12.1.8.1 Business overview
12.1.8.2 Products offered
12.1.8.3 Recent developments
12.1.9 Business overview
12.1.9.1 Business overview
12.1.9.2 Products offered
12.1.9.3 Recent developments
12.1.10 Business overview
12.1.10.1 Business overview
12.1.10.2 Products offered
12.1.10.3 Recent developments
12.1.11 Business overview
12.1.11.1 Business overview
12.1.11.2 Products offered
12.1.12 DIASORIN S.P.A.
12.1.12.1 Business overview
12.1.12.2 Products offered
12.1.12.3 Recent developments
12.1.13 Business overview
12.1.13.1 Business overview
12.1.13.2 Products offered
12.1.13.3 Recent developments
12.1.14 Business overview
12.1.14.1 Business overview
12.1.14.2 Products offered
12.1.15 BIOMÉRIEUX SA
12.1.15.1 Business overview
12.1.15.2 Products offered
12.1.15.3 Recent developments
12.2 START-UP/SME PLAYERS
12.2.1 Products offered
12.2.1.1 Business overview
12.2.1.2 Products offered
12.2.2 QUIDEL CORPORATION
12.2.2.1 Business overview
12.2.2.2 Products offered
12.2.2.3 Recent developments
12.2.3 Business overview
12.2.3.1 Business overview
12.2.3.2 Products offered
12.2.3.3 Recent developments
12.2.4 Business overview
12.2.4.1 Business overview
12.2.4.2 Products offered
12.2.5 CANCER DIAGNOSTICS INC.
12.2.5.1 Business overview
12.2.5.2 Products offered
12.2.5.3 Recent developments
12.2.6 Business overview
12.2.6.1 Business overview
12.2.6.2 Products offered
12.2.7 BIO-RAD LABORATORIES
12.2.7.1 Business overview
12.2.7.2 Products offered
12.2.8 BIO SB
12.2.8.1 Business overview
12.2.8.2 Products offered
12.2.8.3 Recent developments
13 APPENDIX (Page No. - 271)
13.1 DISCUSSION GUIDE
13.2 KNOWLEDGE STORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
13.3 AVAILABLE CUSTOMIZATIONS
13.4 RELATED REPORTS
13.5 AUTHOR DETAILS
LIST OF TABLES (300 Tables)
TABLE 1 INCREASING INCIDENCE OF CANCER, BY REGION, 2020 VS. 2030 VS. 2040 (MILLION)
TABLE 2 PROJECTED INCREASE IN THE GLOBAL NUMBER OF CANCER PATIENTS, 2015 VS. 2018 VS. 2035
TABLE 3 NUMBER OF PATHOLOGISTS PER 10,000 POPULATION, BY COUNTRY, 2018
TABLE 4 IMPORT DATA FOR MAMMOGRAPHY SYSTEMS, BY COUNTRY, 2016—2020 (USD MILLION)
TABLE 5 EXPORT DATA FOR MAMMOGRAPHY SYSTEMS, BY COUNTRY, 2016—2020 (USD MILLION)
TABLE 6 IMPORT DATA FOR MRI SYSTEMS, BY COUNTRY, 2016—2020 (USD MILLION)
TABLE 7 EXPORT DATA FOR MRI SYSTEMS, BY COUNTRY, 2016—2020 (USD MILLION)
TABLE 8 PRICES OF CANCER DIAGNOSTIC PRODUCTS
TABLE 9 CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 10 MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 11 MARKET FOR CONSUMABLES, BY REGION, 2019–2026 (USD MILLION)
TABLE 12 MARKET FOR ANTIBODIES, BY REGION, 2019–2026 (USD MILLION)
TABLE 13 CANCER DIAGNOSTICS MARKET FOR KITS & REAGENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 14 MARKET FOR PROBES, BY REGION, 2019–2026 (USD MILLION)
TABLE 15 MARKET FOR OTHER CONSUMABLES, BY REGION, 2019–2026 (USD MILLION)
TABLE 16 MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 17 MARKET FOR INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 18 MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 19 MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 20 CANCER DIAGNOSTICS MARKET FOR SLIDE STAINING SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 21 MARKET FOR TISSUE PROCESSING SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 22 MARKET FOR CELL PROCESSORS, BY REGION, 2019–2026 (USD MILLION)
TABLE 23 MARKET FOR PCR INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 24 MARKET FOR NGS INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 25 MARKET FOR MICROARRAYS, BY REGION, 2019–2026 (USD MILLION)
TABLE 26 CANCER DIAGNOSTICS MARKET FOR OTHER PATHOLOGY-BASED INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 27 MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 28 MARKET FOR IMAGING INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 29 MARKET FOR CT SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 30 MARKET FOR ULTRASOUND SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 31 MARKET FOR MRI SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 32 CANCER DIAGNOSTICS MARKET FOR MAMMOGRAPHY SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 33 MARKET FOR NUCLEAR IMAGING SYSTEMS, BY REGION, 2019–2026 (USD MILLION)
TABLE 34 MARKET FOR BIOPSY INSTRUMENTS, BY REGION, 2019–2026 (USD MILLION)
TABLE 35 MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 36 MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 37 MARKET FOR IVD TESTING, BY REGION, 2019–2026 (USD MILLION)
TABLE 38 MARKET FOR POLYMERASE CHAIN REACTION, BY REGION, 2019–2026 (USD MILLION)
TABLE 39 MARKET FOR IN SITU HYBRIDIZATION, BY REGION, 2019–2026 (USD MILLION)
TABLE 40 CANCER DIAGNOSTICS MARKET FOR IMMUNOHISTOCHEMISTRY, BY REGION, 2019–2020 (USD MILLION)
TABLE 41 MARKET FOR NEXT-GENERATION SEQUENCING, BY REGION, 2019–2026 (USD MILLION)
TABLE 42 MARKET FOR IMMUNOASSAY, BY REGION, 2019–2026 (USD MILLION)
TABLE 43 MARKET FOR MICROARRAY, BY REGION, 2019–2026 (USD MILLION)
TABLE 44 MARKET FOR FLOW CYTOMETRY, BY REGION, 2019–2026 (USD MILLION)
TABLE 45 CANCER DIAGNOSTIC MARKET FOR OTHER IVD TECHNOLOGIES, BY REGION, 2019–2026 (USD MILLION)
TABLE 46 MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 47 MARKET FOR IMAGING TECHNOLOGIES, BY REGION, 2019–2026 (USD MILLION)
TABLE 48 CANCER DIAGNOSTICS MARKET FOR MAGNETIC RESONANCE IMAGING, BY REGION, 2019–2026 (USD MILLION)
TABLE 49 MARKET FOR COMPUTED TOMOGRAPHY, BY REGION, 2019–2026 (USD MILLION)
TABLE 50 MARKET FOR NUCLEAR IMAGING, BY REGION, 2019–2026 (USD MILLION)
TABLE 51 MARKET FOR MAMMOGRAPHY, BY REGION, 2019–2026 (USD MILLION)
TABLE 52 MARKET FOR ULTRASOUND, BY REGION, 2019–2026 (USD MILLION)
TABLE 53 CANCER DIAGNOSTICS MARKET FOR BIOPSIES, BY REGION, 2019–2026 (USD MILLION)
TABLE 54 MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 55 BREAST CANCER INCIDENCE, BY REGION, 2020 VS. 2040
TABLE 56 MARKET FOR BREAST CANCER, BY REGION, 2019–2026 (USD MILLION)
TABLE 57 LUNG CANCER INCIDENCE, BY REGION, 2020 VS. 2040
TABLE 58 CANCER DIAGNOSTICS MARKET FOR LUNG CANCER, BY REGION, 2019–2026 (USD MILLION)
TABLE 59 COLORECTAL CANCER INCIDENCE, BY REGION, 2020 VS. 2040
TABLE 60 MARKET FOR COLORECTAL CANCER, BY REGION, 2019–2026 (USD MILLION)
TABLE 61 MELANOMA INCIDENCE, BY REGION, 2020 VS. 2040
TABLE 62 MARKET FOR MELANOMA, BY REGION, 2019–2026 (USD MILLION)
TABLE 63 CANCER DIAGNOSTICS MARKET FOR OTHER CANCERS, BY REGION, 2019–2026 (USD MILLION)
TABLE 64 MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 65 MARKET FOR HOSPITALS, BY REGION, 2019–2026 (USD MILLION)
TABLE 66 MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2019–2026 (USD MILLION)
TABLE 67 CANCER DIAGNOSTICS MARKET, BY REGION, 2019–2026 (USD MILLION)
TABLE 68 NORTH AMERICA: MARKET, BY COUNTRY, 2019–2026 (USD MILLION)
TABLE 69 NORTH AMERICA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 70 NORTH AMERICA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 71 NORTH AMERICA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 72 NORTH AMERICA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 73 NORTH AMERICA: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 74 NORTH AMERICA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 75 NORTH AMERICA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 76 NORTH AMERICA: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 77 NORTH AMERICA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 78 NORTH AMERICA: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 79 US: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 80 US: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 81 US: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 82 US: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 83 US: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 84 US: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 85 US: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 86 US: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 87 US: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 88 US: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 89 US: CANCER DIAGNOSTICS MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 90 CANADA: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 91 CANADA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 92 CANADA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 93 CANADA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 94 CANADA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 95 CANADA: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 96 CANADA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 97 CANADA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 98 CANADA: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 99 CANADA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 100 CANADA: CANCER DIAGNOSTICS MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 101 EUROPE: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2019–2026 (USD MILLION)
TABLE 102 EUROPE: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 103 EUROPE: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 104 EUROPE: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 105 EUROPE: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 106 EUROPE: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 107 EUROPE: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 108 EUROPE: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 109 EUROPE: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 110 EUROPE: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 111 EUROPE: CANCER DIAGNOSTICS MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 112 GERMANY: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 113 GERMANY: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 114 GERMANY: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 115 GERMANY: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 116 GERMANY: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 117 GERMANY: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 118 GERMANY: CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 119 GERMANY: CANCER DIAGNOSTIC MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 120 GERMANY: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 121 GERMANY: CANCER DIAGNOSTIC MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 122 GERMANY: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 123 UK: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 124 UK: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 125 UK: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 126 UK: CANCER DIAGNOSTIC MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 127 UK: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 128 UK: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 129 UK: CANCER DIAGNOSTIC MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 130 UK: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 131 UK: CANCER DIAGNOSTIC MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 132 UK: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 133 FRANCE: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 134 FRANCE: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 135 FRANCE: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 136 FRANCE: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 137 FRANCE: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 138 FRANCE: CANCER DIAGNOSTIC MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 139 FRANCE: CANCER DIAGNOSTICS MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 140 FRANCE: CANCER DIAGNOSTIC MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 141 FRANCE: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 142 FRANCE: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 143 FRANCE: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 144 ITALY: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 145 ITALY: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 146 ITALY: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 147 ITALY: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 148 ITALY: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 149 ITALY: CANCER DIAGNOSTIC MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 150 ITALY: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 151 ITALY: CANCER DIAGNOSTIC MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 152 ITALY: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 153 ITALY: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 154 ITALY: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 155 SPAIN: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 156 SPAIN: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 157 SPAIN: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 158 SPAIN: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 159 SPAIN: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 160 SPAIN: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 161 SPAIN: CANCER DIAGNOSTIC MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 162 SPAIN: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 163 SPAIN: CANCER DIAGNOSTIC MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 164 SPAIN: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 165 SPAIN: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 166 LUNG CANCER INCIDENCE IN KEY ROE COUNTRIES, 2018 VS. 2025
TABLE 167 LIVER CANCER INCIDENCE IN KEY ROE COUNTRIES, 2018 VS. 2025
TABLE 168 BREAST CANCER INCIDENCE IN KEY ROE COUNTRIES, 2018 VS. 2025
TABLE 169 ROE: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 170 ROE: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 171 ROE: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 172 ROE: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 173 ROE: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 174 ROE: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 175 ROE: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 176 ROE: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 177 ROE: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 178 ROE: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 179 ASIA PACIFIC: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2019–2026 (USD MILLION)
TABLE 180 ASIA PACIFIC: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 181 ASIA PACIFIC: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 182 ASIA PACIFIC: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 183 ASIA PACIFIC: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 184 ASIA PACIFIC: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 185 ASIA PACIFIC: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 186 ASIA PACIFIC: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 187 ASIA PACIFIC: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 188 ASIA PACIFIC: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 189 ASIA PACIFIC: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 190 CHINA: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 191 CHINA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 192 CHINA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 193 CHINA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 194 CHINA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 195 CHINA: CANCER DIAGNOSTIC MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 196 CHINA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 197 CHINA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 198 CHINA: CANCER DIAGNOSTIC MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 199 CHINA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 200 CHINA: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 201 JAPAN: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 202 JAPAN: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 203 JAPAN: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 204 JAPAN: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 205 JAPAN: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 206 JAPAN: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 207 JAPAN: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 208 JAPAN: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 209 JAPAN: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 210 JAPAN: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 211 JAPAN: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 212 INDIA: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 213 INDIA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 214 INDIA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 215 INDIA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 216 INDIA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 217 INDIA: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 218 INDIA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 219 INDIA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 220 INDIA: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 221 INDIA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 222 INDIA: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 223 INCIDENCE OF CANCER IN ROAPAC COUNTRIES
TABLE 224 ROAPAC: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 225 ROAPAC: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 226 ROAPAC: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 227 ROAPAC: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 228 ROAPAC: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 229 ROAPAC: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 230 ROAPAC: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 231 ROAPAC: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 232 ROAPAC: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 233 ROAPAC: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 234 ROW: CANCER DIAGNOSTICS MARKET, BY REGION, 2019–2026 (USD MILLION)
TABLE 235 ROW: MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 236 ROW: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 237 ROW: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 238 ROW: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 239 ROW: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 240 ROW: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 241 ROW: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 242 ROW: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 243 ROW: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 244 ROW: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 245 LUNG CANCER INCIDENCE IN KEY LATIN AMERICAN COUNTRIES, 2018 VS. 2025
TABLE 246 LIVER CANCER INCIDENCE IN KEY LATIN AMERICAN COUNTRIES, 2018 VS. 2025
TABLE 247 BREAST CANCER INCIDENCE IN KEY LATIN AMERICAN COUNTRIES, 2018 VS. 2025
TABLE 248 LATIN AMERICA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 249 LATIN AMERICA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 250 LATIN AMERICA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 251 LATIN AMERICA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 252 LATIN AMERICA: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 253 LATIN AMERICA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 254 LATIN AMERICA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 255 LATIN AMERICA: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 256 LATIN AMERICA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 257 LATIN AMERICA: MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 258 AFRICA: CANCER INCIDENCE, BY TYPE OF CANCER, 2018 VS. 2025
TABLE 259 MIDDLE EAST & AFRICA: CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2019–2026 (USD MILLION)
TABLE 260 MIDDLE EAST & AFRICA: MARKET FOR CONSUMABLES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 261 MIDDLE EAST & AFRICA: MARKET FOR INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 262 MIDDLE EAST & AFRICA: MARKET FOR PATHOLOGY-BASED INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 263 MIDDLE EAST & AFRICA: MARKET FOR IMAGING INSTRUMENTS, BY TYPE, 2019–2026 (USD MILLION)
TABLE 264 MIDDLE EAST & AFRICA: MARKET, BY TECHNOLOGY, 2019–2026 (USD MILLION)
TABLE 265 MIDDLE EAST & AFRICA: MARKET FOR IVD TESTING, BY TYPE, 2019–2026 (USD MILLION)
TABLE 266 MIDDLE EAST & AFRICA: MARKET FOR IMAGING TECHNOLOGIES, BY TYPE, 2019–2026 (USD MILLION)
TABLE 267 MIDDLE EAST & AFRICA: MARKET, BY APPLICATION, 2019–2026 (USD MILLION)
TABLE 268 MIDDLE EAST & AFRICA: CANCER DIAGNOSTICS MARKET, BY END USER, 2019–2026 (USD MILLION)
TABLE 269 MARKET FOR IMAGING TECHNOLOGIES: DEGREE OF COMPETITION
TABLE 270 MARKET FOR PATHOLOGY-BASED INSTRUMENTS: DEGREE OF COMPETITION
TABLE 271 KEY PRODUCT LAUNCHES & APPROVALS, JANUARY 2018–APRIL 2021
TABLE 272 KEY DEALS, JANUARY 2018–APRIL 2021
TABLE 273 OTHER KEY DEVELOPMENTS, JANUARY 2018–APRIL 2021
TABLE 274 COMPANY FOOTPRINT
TABLE 275 COMPANY PRODUCT FOOTPRINT
TABLE 276 COMPANY APPLICATION FOOTPRINT
TABLE 277 COMPANY GEOGRAPHICAL FOOTPRINT
TABLE 278 GE HEALTHCARE: BUSINESS OVERVIEW
TABLE 279 BECTON, DICKINSON AND COMPANY: BUSINESS OVERVIEW
TABLE 280 F. HOFFMAN-LA ROCHE LTD.: BUSINESS OVERVIEW
TABLE 281 DANAHER CORPORATION: BUSINESS OVERVIEW
TABLE 282 THERMO FISHER SCIENTIFIC: BUSINESS OVERVIEW
TABLE 283 ABBOTT LABORATORIES: BUSINESS OVERVIEW
TABLE 284 QIAGEN N.V.: BUSINESS OVERVIEW
TABLE 285 AGILENT TECHNOLOGIES INC.: BUSINESS OVERVIEW
TABLE 286 ILLUMINA INC.: BUSINESS OVERVIEW
TABLE 287 SIEMENS HEALTHINEERS AG: BUSINESS OVERVIEW
TABLE 288 DIASORIN S.P.A.: BUSINESS OVERVIEW
TABLE 289 MYRIAD GENETICS INC.: BUSINESS OVERVIEW
TABLE 290 HOLOGIC, INC.: BUSINESS OVERVIEW
TABLE 291 BIOMÉRIEUX SA: BUSINESS OVERVIEW
TABLE 292 FUJIFILM HOLDINGS CORPORATION: BUSINESS OVERVIEW
TABLE 293 QUIDEL CORPORATION: BUSINESS OVERVIEW
TABLE 294 EXACT SCIENCES: BUSINESS OVERVIEW
TABLE 295 BIOCARTIS NV: BUSINESS OVERVIEW
TABLE 296 CANCER DIAGNOSTICS INC.: BUSINESS OVERVIEW
TABLE 297 AMOY DIAGNOSTICS CO. LTD.: BUSINESS OVERVIEW
TABLE 298 BIO-RAD LABORATORIES: BUSINESS OVERVIEW
TABLE 299 BIO SB: BUSINESS OVERVIEW
TABLE 300 VELA DIAGNOSTICS: BUSINESS OVERVIEW
LIST OF FIGURES (48 Figures)
FIGURE 1 RESEARCH DESIGN
FIGURE 2 PRIMARY SOURCES
FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
FIGURE 4 SUPPLY-SIDE MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS
FIGURE 5 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
FIGURE 6 TOP-DOWN APPROACH
FIGURE 7 DATA TRIANGULATION METHODOLOGY
FIGURE 8 CRITERIA IMPACTING THE GLOBAL ECONOMY
FIGURE 9 RECOVERY SCENARIO OF THE GLOBAL ECONOMY
FIGURE 10 CANCER DIAGNOSTICS MARKET, BY PRODUCT, 2021 VS. 2026 (USD BILLION)
FIGURE 11 MARKET, BY TECHNOLOGY, 2021 VS. 2026 (USD BILLION)
FIGURE 12 MARKET, BY APPLICATION, 2021 VS. 2026 (USD BILLION)
FIGURE 13 MARKET, BY END USER, 2021 VS. 2026 (USD BILLION)
FIGURE 14 MARKET, BY REGION, 2021 VS. 2026 (USD BILLION)
FIGURE 15 INCREASING INCIDENCE OF CANCER TO DRIVE MARKET GROWTH DURING THE FORECAST PERIOD
FIGURE 16 INSTRUMENTS SEGMENT ACCOUNTED FOR THE LARGEST MARKET SHARE IN 2020
FIGURE 17 US DOMINATED THE NORTH AMERICAN MARKET IN 2020
FIGURE 18 CHINA TO REGISTER THE HIGHEST GROWTH DURING THE FORECAST PERIOD
FIGURE 19 CANCER DIAGNOSTICS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
FIGURE 20 RISING NUMBER OF PERSONALIZED MEDICINES, 2008–2020
FIGURE 21 MAJOR VALUE IS ADDED DURING THE MANUFACTURING & ASSEMBLY PHASES
FIGURE 22 DISTRIBUTION—A STRATEGY PREFERRED BY PROMINENT COMPANIES
FIGURE 23 510(K) APPROVAL PROCESS
FIGURE 24 NORTH AMERICA: MARKET SNAPSHOT
FIGURE 25 ASIA PACIFIC: MARKET SNAPSHOT
FIGURE 26 KEY DEVELOPMENTS IN THE MARKET, JANUARY 2018–APRIL 2021
FIGURE 27 MARKET EVOLUTION MATRIX: JANUARY 2018 TO APRIL 2021
FIGURE 28 VENDOR DIVE: MARKET
FIGURE 29 VENDOR DIVE MATRIX FOR SMES & START-UPS: CANCER DIAGNOSTICS MARKET
FIGURE 30 GE HEALTHCARE: COMPANY SNAPSHOT (2020)
FIGURE 31 BECTON, DICKINSON AND COMPANY: COMPANY SNAPSHOT (2020)
FIGURE 32 F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT (2020)
FIGURE 33 DANAHER CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 34 THERMO FISHER SCIENTIFIC: COMPANY SNAPSHOT (2020)
FIGURE 35 ABBOTT LABORATORIES: COMPANY SNAPSHOT (2020)
FIGURE 36 QIAGEN N.V.: COMPANY SNAPSHOT (2020)
FIGURE 37 AGILENT TECHNOLOGIES INC.: COMPANY SNAPSHOT (2020)
FIGURE 38 ILLUMINA, INC.: COMPANY SNAPSHOT (2020)
FIGURE 39 SIEMENS HEALTHINEERS: COMPANY SNAPSHOT (2020)
FIGURE 40 DIASORIN S.P.A.: COMPANY SNAPSHOT (2019)
FIGURE 41 MYRIAD GENETICS INC.: COMPANY SNAPSHOT (2020)
FIGURE 42 HOLOGIC INC.: COMPANY SNAPSHOT (2020)
FIGURE 43 BIOMÉRIEUX SA: COMPANY SNAPSHOT (2020)
FIGURE 44 FUJIFILM HOLDINGS CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 45 QUIDEL CORPORATION: COMPANY SNAPSHOT (2020)
FIGURE 46 EXACT SCIENCES: COMPANY SNAPSHOT (2020)
FIGURE 47 BIOCARTIS NV: COMPANY SNAPSHOT (2020)
FIGURE 48 BIO-RAD LABORATORIES: COMPANY SNAPSHOT (2020)
The study involved four major activities in estimating the current size of the cancer diagnostics market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation procedures were used to estimate the market size of the segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
Primary Research
Extensive primary research was conducted after acquiring knowledge about the global market scenario through secondary research. Primary interviews were conducted from both the demand (hospitals and diagnostic laboratories) and supply sides (cancer diagnostic manufacturers and distributors).
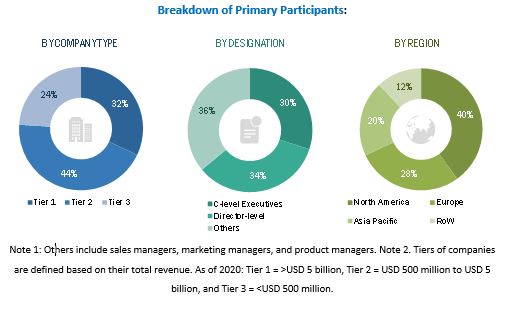
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the cancer diagnostics market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and markets have been identified through extensive secondary research
- The industry’s supply chain and market size, in terms of value, have been determined through primary and secondary research processes
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Data Triangulation
After arriving at the overall market size-using the market size estimation processesthe market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, the data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the cancer diagnostics industry.
Report Objectives
- To define, describe, and forecast the global cancer diagnostics market based on product, technology, application, end user, and region
- To provide detailed information regarding the major factors influencing the growth of the market (drivers, restraints, opportunities, and industry-specific challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of market segments with respect to four main regions—North America, Europe, Asia Pacific and the Rest of the World (RoW)2
- To strategically profile key players and comprehensively analyze their product portfolios, market shares, and core competencies3
- To track and analyze competitive developments such as acquisitions, new product launches, expansions, regulatory approvals, and agreements in the cancer diagnostics market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
Product Analysis:
Geographic Analysis
-
- Product matrix, which gives a detailed comparison of the product portfolios of each company
- Further breakdown of the Rest of the World cancer diagnostics market into Latin America, the Middle East, and Africa cancer diagnostics market into specific countries and further breakdown of the European cancer diagnostics market into specific countries



 Generating Response ...
Generating Response ...









Growth opportunities and latent adjacency in Cancer Diagnostics Market
Need accurate market data of Cancer Diagnostics Market Size, share and Forecasts upto 2030
What are the growth expectations for the leading players in the global Cancer Diagnostics Market?
Which factors are proving to be the growth boosters for the North American region of the Cancer Diagnostics Market?