The objective of the study is to analyze the key market dynamics, such as drivers, opportunities, restraints, challenges, and key player strategies. To track company developments such as acquisitions, product launches, expansions, agreements, and partnerships of the leading players, the competitive landscape of the rapid microbiology testing market to analyze market players on various parameters within the broad categories of business and product strategy. Top-down and bottom-up approaches were used to estimate the market size. To estimate the market size of segments and subsegments, the market breakdown and data triangulation were used.
The four steps involved in estimating the market size are
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information as well as assess prospects.
The following is a breakdown of the primary respondents:
Breakdown of Primary Participants:
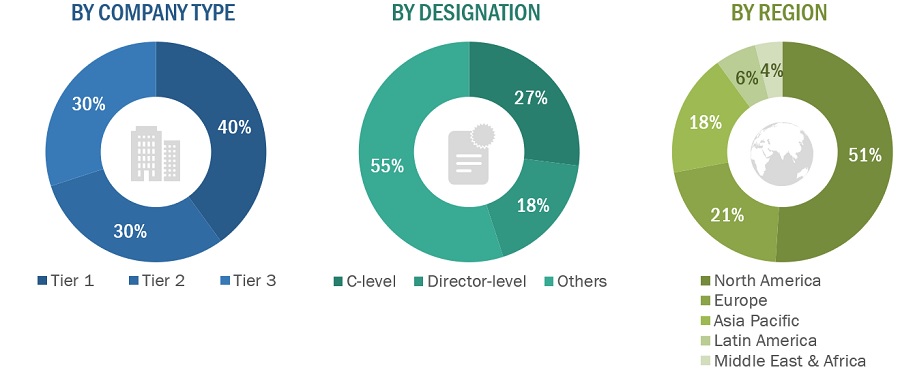
Note 1: Others include sales managers, marketing managers, and product managers.
Note 2: Companies are classified into tiers based on their total revenues. As of 2022/2023, Tier 1 = >USD 100 million, Tier 2 = USD 10 million to USD 100 million, and Tier 3 = <USD 10 million.
To know about the assumptions considered for the study, download the pdf brochure
|
COMPANY NAME
|
DESIGNATION
|
|
Abbott
|
Senior Manager
|
|
Becton, Dickinson and Company
|
Sales Executive
|
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the rapid microbiology testing market's total size. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
-
The key players in the industry have been identified through extensive secondary research
-
The revenues generated by leading players operating in the rapid microbiology testing market have been determined through primary and secondary research.
-
All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Global Rapid microbiology testing Market Size: Bottom Up Approach
To know about the assumptions considered for the study, Request for Free Sample Report
Global Rapid Microbiology Testing Market Size: Top-Down Approach

Data Triangulation
After arriving at the overall market size by applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Rapid microbiology testing products are used to rapidly detect, screen, enumerate, and identify microbes and microbial contaminants causing or spreading diseases. These testing procedures include microbial methods that give sensitive and accurate results faster than conventional microbial testing methods.
Key Stakeholders
-
Senior Management
-
End User
-
Finance/Procurement Department
-
R&D Department
Report Objectives
-
To define, describe, segment, and forecast the rapid microbiology testing market by product, method, application, and region
-
To provide detailed information regarding the major factors influencing the market growth (drivers, restraints, opportunities, and challenges)
-
To analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
-
To forecast the size of the market segments with respect to six regions: North America, Europe, the Asia Pacific, Middle East & Africa, Latin America, and GCC countries
-
To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
-
To track and analyze company developments such as acquisitions, collaborations, partnerships, agreements, and product launches & approvals in the rapid microbiology testing market
-
To benchmark players within the market using the Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offerings
Available Customizations
MarketsandMarkets offers the following customizations for this market report.
Country Information
-
Additional country-level analysis of the rapid microbiology testing market
Company profiles
-
Additional five company profiles of players operating in the rapid microbiology testing market.
Product Analysis
-
Product matrix, which provides a detailed comparison of the product portfolio of each company in the rapid microbiology testing market



Growth opportunities and latent adjacency in Rapid Microbiology Testing Market