The study involved major activities in estimating the current market size for the therapeutic drug monitoring market. Exhaustive secondary research was done to collect information on the therapeutic drug monitoring industry. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, such as top-down and bottom-up, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were used to estimate the market size of the segments and subsegments of the therapeutic drug monitoring market.
Secondary Research
The market for the companies offering therapeutic drug monitoring solutions is arrived at by secondary data available through paid and unpaid sources, analyzing the product portfolios of the major companies in the ecosystem, and rating the companies by their performance and quality. Various sources were referred to in the secondary research process to identify and collect information for this study. The secondary sources include annual reports, press releases, investor presentations of companies, white papers, journals, certified publications, and articles from recognized authors, directories, and databases.
In the secondary research process, various secondary sources were referred to for identifying and collecting information related to the study. Secondary sources included annual reports, press releases, and investor presentations of blockchain vendors, forums, certified publications, and whitepapers. The secondary research was used to obtain critical information on the industry’s value chain, the total pool of key players, market classification, and segmentation from the market and technology-oriented perspectives.
Primary Research
In the primary research process, various primary sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side included industry experts, such as Chief Executive Officers (CEOs), Vice Presidents (VPs), marketing directors, technology and innovation directors, and related key executives from various key companies and organizations operating in the blockchain market. After the complete market engineering (calculations for market statistics, market breakdown, market size estimations, market forecasting, and data triangulation), extensive primary research was conducted to gather information and verify and validate the critical numbers arrived at. Primary research was also conducted to identify the segmentation types, industry trends, competitive landscape of therapeutic drug monitoring solutions offered by various market players, and key market dynamics, such as drivers, restraints, opportunities, challenges, industry trends, and key player strategies.
In the complete market engineering process, the top-down and bottom-up approaches were extensively used, along with several data triangulation methods, to perform the market estimation and market forecasting for the overall market segments and subsegments listed in this report. Extensive qualitative and quantitative analysis was performed on the complete market engineering process to list the key information/insights throughout the report.
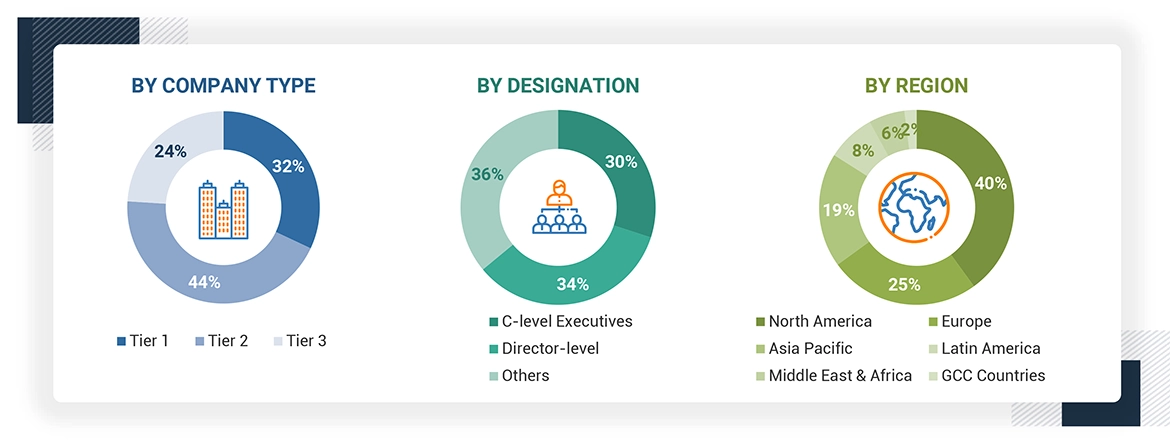
*C-level primaries include CEOs, CFOs, COOs, and VPs.
*Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
Note: Companies are classified into tiers based on their total revenue. As of 2023, Tier 1 = >USD 10.00 billion, Tier 2 = USD 1.00 billion to USD 10.00 billion, and Tier 3 = < USD 1.00 billion.
Source: MarketsandMarkets Analysis
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the cell culture market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
After arriving at the overall market size using the market size estimation processes explained above, the market was split into several segments and subsegments. The data triangulation and market breakup procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Therapeutic drug monitoring (TDM) measures the concentration of specific drugs in body fluids, which helps manage drug therapy to cure, alleviate, or prevent diseases. TDM is used mainly for monitoring drugs with narrow therapeutic ranges, drugs with marked pharmacokinetic variability, medications for which target concentrations are difficult to monitor, and drugs known to cause therapeutic and adverse effects.
Stakeholders
-
Therapeutic Drug Monitoring Product Manufacturers
-
Healthcare Service Providers
-
Research Associations
-
Research Institutes
-
Venture Capitalists and Investors
-
Various Research and Consulting Companies
-
Pharmaceutical and Biopharmaceutical Companies
-
Diagnostic Associations
-
Clinical Laboratories
-
Suppliers/Distributors of Therapeutic Drug Monitoring Products and Consumables
-
Hospitals
-
Government and Non-governmental Regulatory Authorities
Report Objectives
-
To define, describe, and forecast the therapeutic drug monitoring market based on – product, technology, drug class, therapeutic area, specimen, end user, and region.
-
To provide detailed information about the major factors (drivers, opportunities, restraints, and challenges) influencing the growth of the market.
-
To analyze opportunities for stakeholders by identifying the high-growth segments of the market
-
To forecast the size of the market segments with respect to six main regions: North America, Europe, Asia Pacific, Latin America, the Middle East & Africa, and GCC Countries.
-
To analyze subsegments of the market with respect to individual growth trends, prospects, and contributions to the overall market.
-
To profile the key players and comprehensively analyze their market sizes and core competencies
-
To track and analyze competitive developments such as product enhancements and new product launches, acquisitions, and partnerships & collaborations in the market globally.



Growth opportunities and latent adjacency in Therapeutic Drug Monitoring Market