This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the Intracardiac echocardiography (ICE) market. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in product therapy markets. The primary sources from the demand side include medical OEMs, Analytical instrument OEMs, CDMOs, and service providers, among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
A breakdown of the primary respondents is provided below:
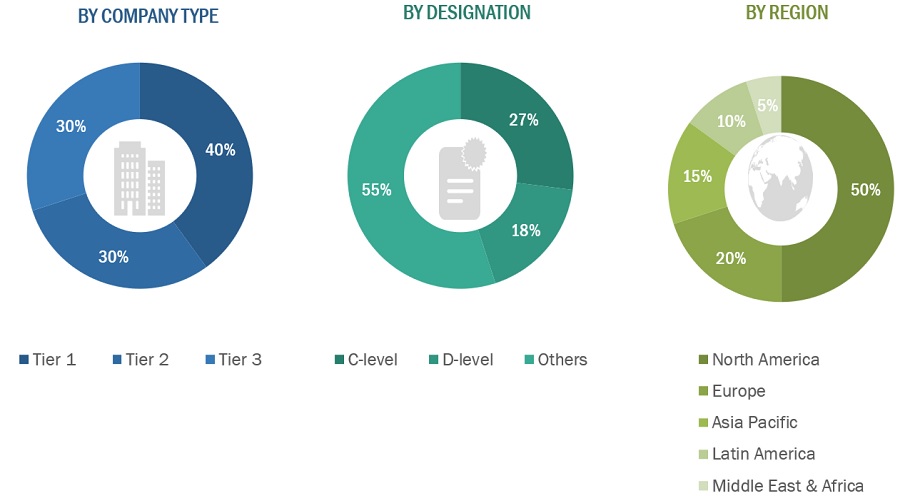
*Others include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
Note: Companies are classified into tiers based on their total revenue. As of 2021, Tier 1 = >USD 2 billion, Tier 2 = USD 50 million to USD 2 billion, and Tier 3 = <USD 50 million.
To know about the assumptions considered for the study, download the pdf brochure
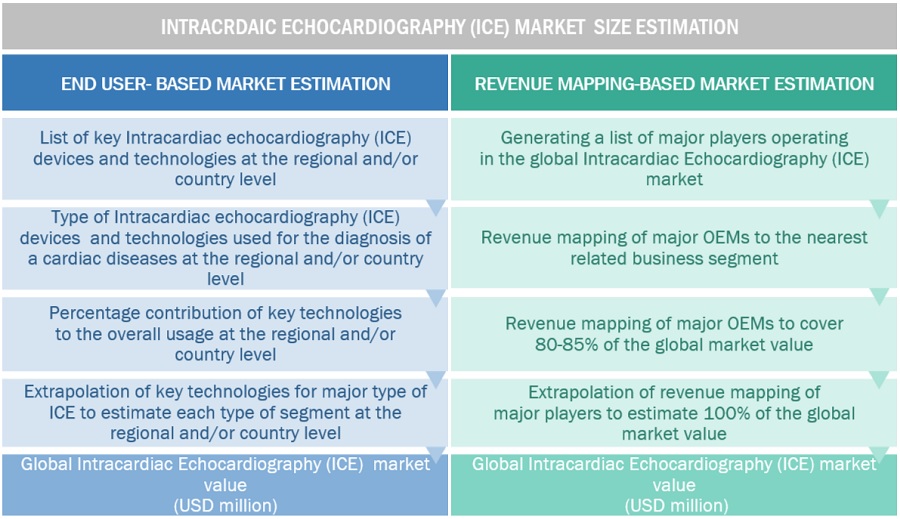
Market Estimation Methodology
In this report, the intracardiac echocardiography (ICE) market size was determined using the revenue analysis of leading players. For this purpose, key players in the market were identified, and their revenues from the intracardiac echocardiography (ICE) market business were determined through various insights gathered during the primary and secondary research phases. Secondary research included the study of the annual and financial reports of the top market players. In contrast, primary research included extensive interviews with key opinion leaders, such as CEOs, directors, and key marketing executives.
To calculate the global market value, segmental revenues were calculated based on the revenue mapping of major solution/service providers. This process involved the following steps:
-
Generating a list of major global players operating in the intracardiac echocardiography (ICE) market.
-
Mapping annual revenues generated by major global players from the intracardiac echocardiography (ICE) market (or nearest reported business unit/product category).
-
Revenue mapping of key players to cover a major share of the global market, as of 2023
-
Extrapolating the global value of the intracardiac echocardiography (ICE) market.
Global Intracardiac echocardiography (ICE) Market: Bottom-Up Approach

To know about the assumptions considered for the study, Request for Free Sample Report
Global Intracardiac echocardiography (ICE) Market : Top-Down Approach

Data Triangulation
After arriving at the overall market size from the market size estimation process explained above, the intracardiac echocardiography (ICE) market was split into segments and subsegments. Data triangulation and market breakdown procedures were employed to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the intracardiac echocardiography (ICE) market was validated using both top-down and bottom-up approaches.
Market Definition
Intracardiac echocardiography (ICE) is a medical imaging technique uses an ultrasound probe that is inserted into the heart through a catheter to provide a detailed, real-time visualization of the heart's internal structures and functions. This makes it easier to diagnose and treat a variety of cardiac conditions. ICE is frequently used to guide interventional procedures like catheter ablations, device implantations, and structural heart repairs.
Key Stakeholders
-
Manufacturers and distributors of intracardiac echocardiography (ICE) devices
-
Healthcare institutions (Hospitals and surgical centers)
-
Ambulatory surgical centers
-
Independent diagnostic centers
-
Healthcare institutions (hospitals and cardiac centers)
-
Research institutions
-
Research and consulting firms
-
Contract research organizations (CROs) and contract manufacturing organizations (CMOs)
-
Academic medical centers and universities
-
Market research and consulting firms
-
Clinical research organizations
-
Group Purchasing Organizations (GPOs)
-
Academic Medical Centers and Universities
-
Accountable Care Organizations (ACOs)
Objectives of the Study
-
To define, describe, and forecast the intracardiac echocardiography (ICE) market on Product, application and end user.
-
To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges).
-
To strategically analyze the micro markets concerning individual growth trends, prospects, and contributions to the total market.
-
To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders.
-
To profile the key market players and comprehensively analyze their market shares and core competencies.
-
To forecast the revenue of the market segments concerning five main regions, namely, North America (US and Canada), Europe (Germany, France, the UK, Italy, Spain, and Rest of Europe), the Asia Pacific (China, Japan, India, South Korea, Australia, and Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa (GCC Countries and Rest of Middle East & Africa).
-
To track and analyze competitive developments such as new product launches and approvals; agreements, partnerships, expansions, acquisitions; and collaborations in the intracardiac echocardiography (ICE) market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the present global intracardiac echocardiography (ICE) market
Product Analysis
-
Product matrix, which gives a detailed comparison of the product portfolios of the top fifteen companies.
Company Information
-
Detailed analysis and profiling of additional market players (up to 15)
Geographic Analysis
-
Further breakdown of the Rest of Europe's intracardiac echocardiography (ICE) market Russia, Belgium, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal among other
-
Further breakdown of the Rest of Asia Pacific intracardiac echocardiography (ICE) market Singapore, Taiwan, New Zealand, Philippines, Malaysia, and other APAC countries
-
Further breakdown of the Rest of the world intracardiac echocardiography (ICE) market Latin America, MEA, and Africa



Growth opportunities and latent adjacency in Intracardiac Echocardiography Market