This research study was conducted with analysis of both secondary sources and primary research inputs. Secondary sources involved key suppliers & customer filings, investor documents, clinical trial directories, industry associations, among others to collect information for the analysis of the global lipid nanoparticles market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key suppliers and customers, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the lipid nanoparticles market. The secondary sources used for this study include United States Food and Drug Administration (US FDA), National Cancer Institute (NCI), National Institutes of Health (NIH), Organization for Economic Co-operation and Development (OECD), Centers for Medicare and Medicaid Services (CMS), International Monetary Fund (IMF), German Federal Ministry of Education and Research (BMBF), UK Research and Innovation (UKRI), GLOBOCAN, Indian Council of Medical Research (ICMR), Korea Advanced Institute of Science and Technology (KAIST), Korea Drug Development Fund (KDDF), Centre of Excellence in Convergent Bio-Nano Science and Technology (CBNS), Association of Southeast Asian Nations (ASEAN), International Agency for Research on Cancer (IARC), World Economic Situation and Prospects (WESP), research journals; corporate filings such as annual reports, SEC filings, investor presentations, and financial statements; press releases; trade, business, professional associations and among others. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
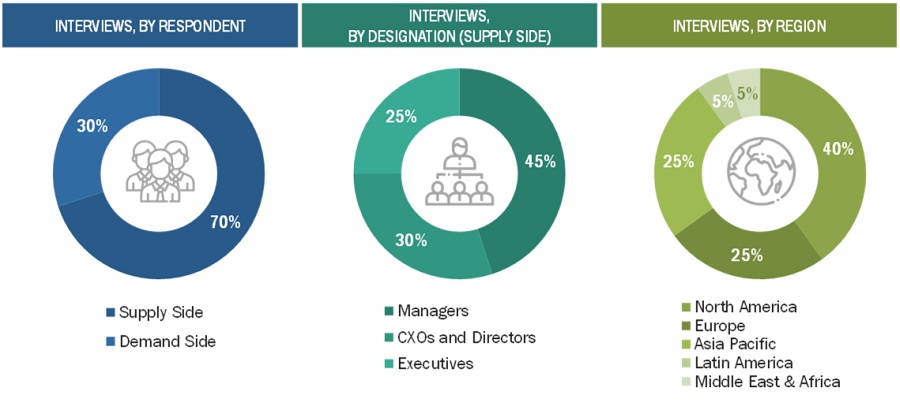
Extensive primary research was conducted after acquiring basic knowledge about the global lipid nanoparticles market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side, such as pharmaceutical and biotechnology companies, CROs, CMOs, and academic & research institutes, and experts from the supply side, such as C-level and D-level executives, product managers, marketing & sales managers of key manufacturers, distributors, and channel partners. These interviews were conducted across six major regions, including the Asia Pacific, North America, Europe, Latin America, Middle East and Africa. Approximately 70% and 30% of the primary interviews were conducted with supply-side and demand-side participants, respectively. This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the lipid nanoparticles market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Bottom-up Approach
-
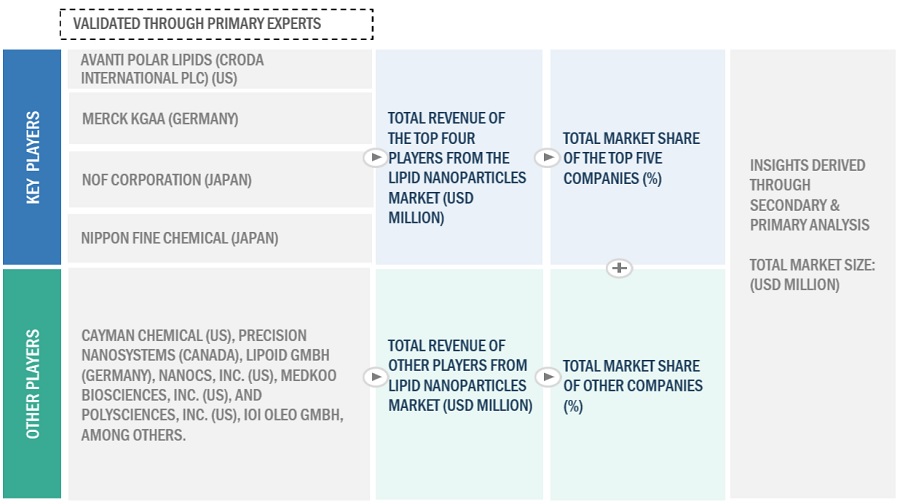
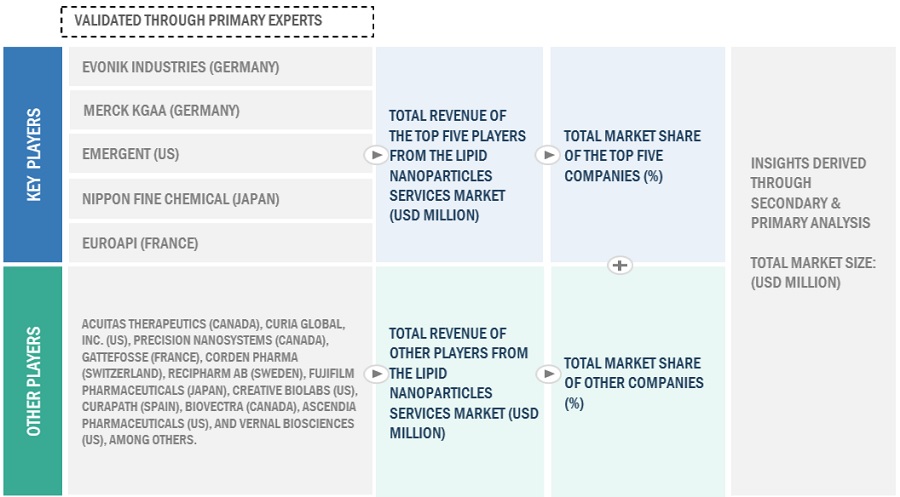
The key players in the industry and market have been identified through extensive secondary research.
-
The revenues generated from the lipid nanoparticles business of leading players have been determined through primary and secondary research to arrive at the overall lipid nanoparticles market.
-
All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
-
Additionally, to arrive at the lipid nanoparticles market for each product, the lipid nanoparticles business shares of leading players for each product / service category were also gathered from secondary sources to the extent available. In some instances, the shares of lipid nanoparticles businesses were ascertained through a detailed analysis of various parameters, including product portfolios and market positioning.
-
Individual shares or revenue estimates were validated through expert interviews.
To know about the assumptions considered for the study, Request for Free Sample Report
Top-down Approach
After arriving at the overall market size from the market size estimation process, the total market was split into several segments and subsegments.

Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
lipid nanoparticles (LNPs) are tiny particles, typically measuring between 20 to 200 nanometers, made from various lipids. They are designed to encapsulate and deliver therapeutic agents, such as nucleic acids (mRNA, siRNA), small Molecule Types, and proteins, to specific cells or tissues. LNPs are composed of a mix of lipids, including phospholipids, cholesterol, and ionizable lipids, which self-assemble to form structures that protect and transport the therapeutic payload.
Stakeholders
-
Academic Research Institutes
-
lipid nanoparticles’ Raw Materials Manufacturers, Vendors, and Distributors
-
Contract Research Organizations (CROs)
-
lipid Nanoparticles Service Providers
-
Market Research and Consulting Firms
-
Pharmaceutical and Biotechnology Companies
-
Regulatory Agencies
-
Venture Capitalists
-
Forensics Labs
-
Government organizations
-
Private research firms
-
Contract development and manufacturing organizations (CDMOs)
Report Objectives
-
To define, describe, and forecast the lipid nanoparticles market based on product, LNP Type, Molecule Type, application, end user, Service Type, end user (Services) and region
-
To provide detailed information regarding the major factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
-
To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall lipid nanoparticles market
-
To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
-
To forecast the size of the market segments with respect to six main regions: North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa
-
To profile the key players in the lipid nanoparticles market and comprehensively analyze their product portfolios, market positions, and core competencies
-
To track and analyze competitive developments in the lipid nanoparticles market, such as acquisitions, product launches, expansions, agreements, partnerships, and collaborations
-
To benchmark players within the lipid nanoparticles market using the ‘Company Evaluation Matrix' framework, which analyzes market players based on various parameters within the broad categories of business strategy and product strategy
1 Micro markets are the further segments and subsegments of the lipid nanoparticles market included in the report
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Portfolio Assessment
-
Product Matrix, which gives a detailed comparison of the product portfolios of the top three companies.
Company Information
-
Detailed analysis and profiling of additional market players (up to three).
Geographical Analysis
-
A further breakdown of the Rest of Asia Pacific lipid nanoparticles market into countries
-
A further breakdown of the Rest of European lipid nanoparticles market into countries
-
A further breakdown of the Rest of Latin American lipid nanoparticles market into countries
-
A further breakdown of the Rest of Middle East lipid nanoparticles market into countries



Growth opportunities and latent adjacency in Lipid Nanoparticles Market