
US Nuclear Medicine Market Size, Growth, Share & Trends Analysis
US Nuclear Medicine Market by Type (SPECT [Tc99m, I-123, Ga-67], PET [F-18], Alpha Emitters, Beta Emitters [Y-90, Lu-177]), Application (Onco, Neuro), Procedure, End User (Imaging Center, Hospital), Radioisotope, Market Share, Growth - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
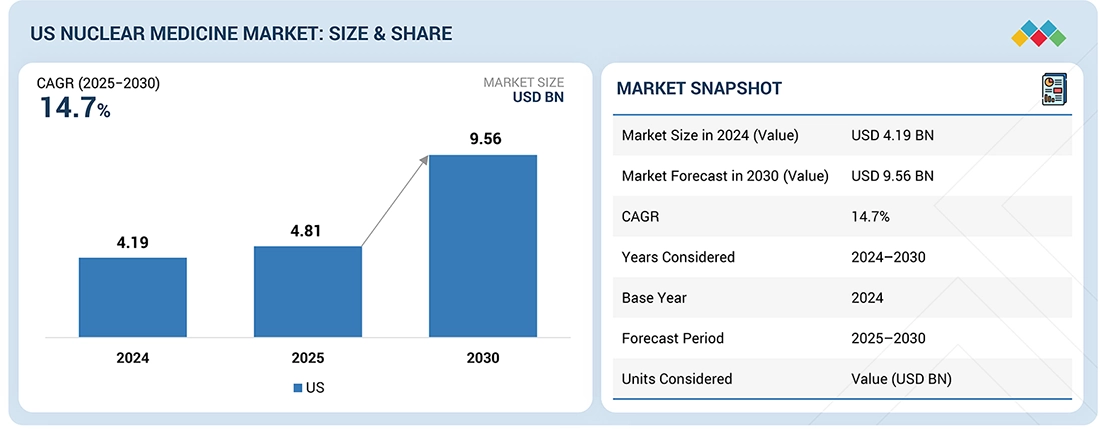
The US Nuclear Medicine market, valued at USD 4.19 billion in 2024, stood at USD 4.81 billion in 2025 and is projected to advance at a resilient CAGR of 14.7% from 2025 to 2030, culminating in a forecasted valuation of USD 9.56 billion by the end of the period. This growth is attributed to the rapid expansion of theranostics and radioligand therapies, growing FDA approval of novel radiopharmaceuticals, and active clinical research at the US hospitals and academic medical centers. Additionally, a favorable reimbursement policy, an investment in the production of domestic radioisotopes, and the progress of automation and AI-based nuclear medicine processes and workflows are contributing to continuous market growth.
KEY TAKEAWAYS
-
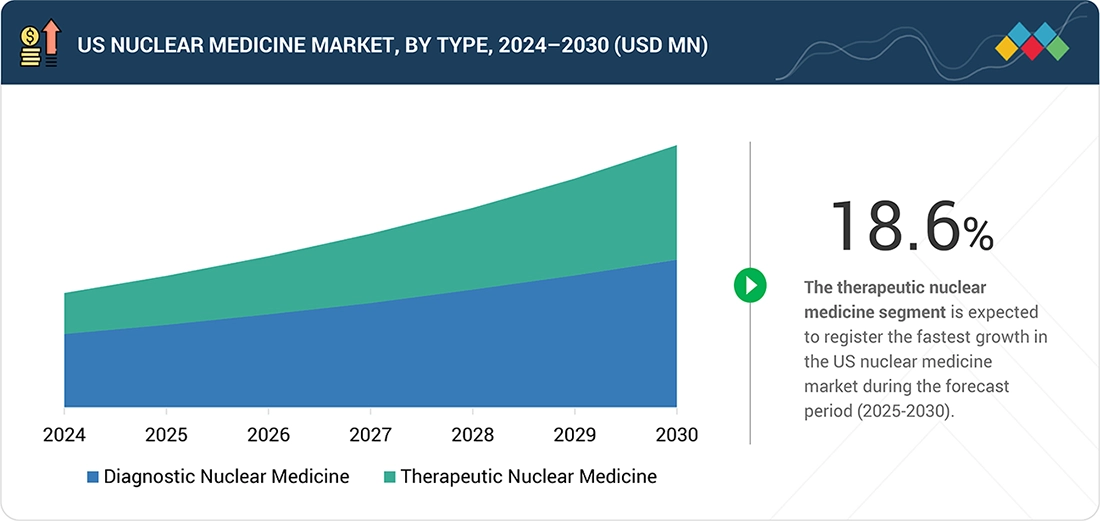
BY TYPEThe therapeutic nuclear medicine segment is expected to register the highest CAGR of 18.6%.
-
BY APPLICATIONThe therapeutic applications segment accounted for the largest share of 64.3% in 2024.
-
BY VOLUME ASSESSMENTThe diagnostic procedures segment is expected to dominate the market.
-
BY END USERThe hospitals segment accounted for the largest share of 54.3% in 2024.
-
COMPETITIVE LANDSCAPEGE HealthCare, Cardinal Health, and Bayer AG are key leaders in the US nuclear medicine market, recognized for their extensive product portfolios, robust data integration capabilities, and strong presence across healthcare ecosystems.
The nuclear medicine market in US is growing because of the continued investment in the development of modern imaging infrastructure and the adoption of high-value radiopharmaceuticals in accordance with the US clinical and regulatory standards. Digital PET/CT, SPECT/CT, and automated radiopharmacy enhancements are increasing accuracy, efficiency, and compliance in healthcare facilities. Additionally, stronger collaboration between US pharmaceutical firms, imaging suppliers, and hospital networks is enabling the more integrated and scalable nuclear medicine solutions.
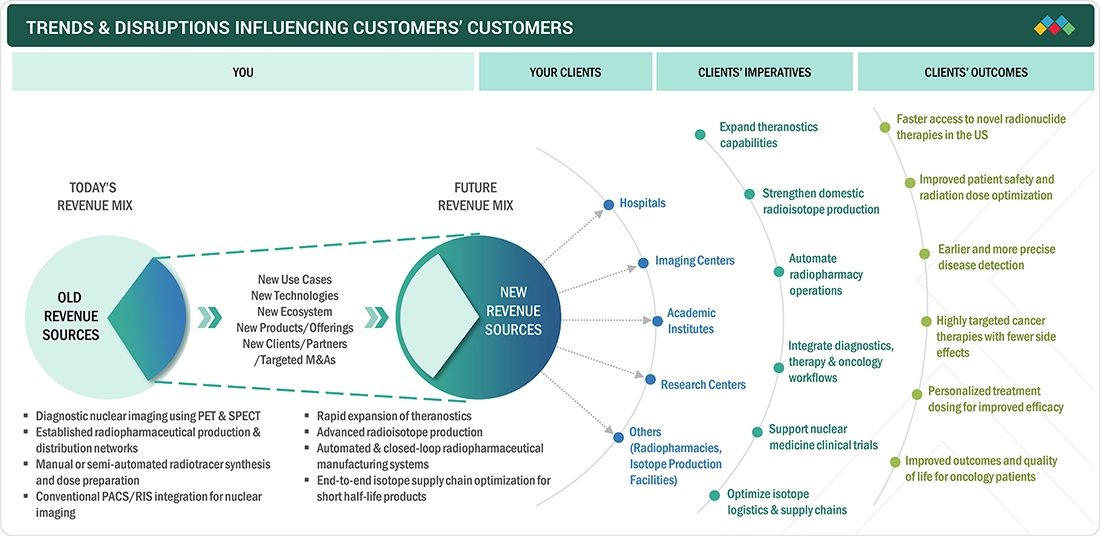
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
In the US, stringent federal and state regulatory controls, the need for precise management of short-half-life radiopharmaceuticals & clinical shift to more complex diagnostic and treatment regimens are driving consumer trends in the field of nuclear medicine. Hospitals, health systems, outpatient imaging networks, and radiopharmacy suppliers in the US are focusing on using hybrid imaging platforms, theranostic workflow, and automated radiopharmacy solutions to achieve safety, accuracy, and throughput demands. These trends are affecting buying patterns, with the providers concentrating on technologies that assist in operational effectiveness, the ability to comply with regulations, cost control, and clinical outcomes aspects that directly impact the competitiveness in the US nuclear medicine ecosystem.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Expansion of theranostics and radioligand therapies in the US

-
Strong reimbursement and healthcare infrastructure
Level
-
Strong reimbursement and healthcare infrastructure
Level
-
Growth in domestic radioisotope production capacity
-
Integration of AI and automation in nuclear medicine workflows
Level
-
Workforce shortages in nuclear medicine specialties
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Expansion of theranostics and radioligand therapies in the US
The expansion of theranostics and radioligand therapies market is a major driving factor in the US nuclear medicine market, as it is transforming nuclear medicine into a therapeutic field. The US is considered a major center for the development and early adoption of FDA-approved radioligand therapies with robust clinical trial efforts, specialty cancer centers, and favorable reimbursement options. This shift is increasing demand for therapeutic radioisotopes and diagnostic radiopharmaceuticals as well as stimulating investment in nuclear pharmacies, production of radioisotopes, and specialized treatment facilities. Consequently, theranostics is boosting the market development and widening the clinical and commercial boundaries of nuclear medicine in the US.
Restraint: Strong reimbursement and healthcare infrastructure
Despite the presence of a well-developed reimbursement system and a developed healthcare infrastructure, which serve as effective drivers to the nuclear medicine market, these elements can also serve as a limiting force to the nuclear medicine market. Nuclear medicine procedures and radiopharmaceuticals are commonly very structured, indication-specific, and subject to constant changes in policies by governmental and even private payers that may restrict the ability to adopt newer and more expensive radiopharmaceuticals. The adverse outcome is that in most situations, coverage decisions do not keep up with FDA approvals, thus establishing delays in the clinical adoption of new nuclear medicine therapy. Moreover, the developed US healthcare system gives high priority to cost control, utilisation control, and value-based care that may limit the amount of procedures and delay the investment decision on the new technologies in nuclear medicine, which would also temper the overall growth of the market.
Opportunity: Growth in domestic radioisotope production capacity
The development of the increased domestic radiological isotope production capacity is a major opportunity for the US nuclear medicine market since it enhances the supply reliability and minimizes the reliance on foreign production. As a response to this, greater investments in US-based cyclotrons, reactors, and other alternative technologies to produce isotopes are contributing to addressing supply vulnerabilities of end users to critical medical radioisotopes. This growth contributes to the uninterrupted supply of both diagnostic and therapeutic products and allows new radiopharmaceuticals to be brought to the market faster, and provides greater security in national supply. Moreover, domestic production opens up the potential of cost-efficiency, public-private collaborations, and accelerated clinical adoption, positioning the US as a more independent and competitive nuclear medicine market.
Challenge: Workforce shortages in nuclear medicine specialties
The shortages of the workforce in the nuclear medicine specialties are the central challenge to the US nuclear medicine market because the field requires highly trained nuclear medicine technologists, radiopharmacists, medical physicists, and specially trained physicians. An ageing workforce, fewer training opportunities, and a growing clinical complexity, as new theranostic and radioligand therapies gain traction, are aggravating the problem of talent shortage around the US. Consequently, healthcare providers are limited in expanding nuclear medicine coverage, using modern technologies, and staying in line with regulatory requirements. Such restrictions in the workforce postponed the procedure volume, augmented the costs of operations, and decelerated the development of the capacity of nuclear medicine in the country.
US NUCLEAR MEDICINE MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Production and supply of PET and SPECT radioisotopes and radiopharmaceuticals for oncology, cardiology, and neurology imaging across the US. | Reliable domestic isotope availability, high diagnostic accuracy, and consistent imaging performance. |
 |
US-based nuclear pharmacy network providing preparation and just-in-time distribution of short-half-life radiopharmaceuticals. | Timely radiotracer delivery, reduced operational burden for providers, and uninterrupted diagnostic workflows. |
 |
Manufacturing and US distribution of diagnostic and therapeutic radiopharmaceuticals, including PET and SPECT radioisotopes. | Stable isotope supply, high product quality, and expanded access to nuclear medicine procedures nationwide. |
 |
Radiopharmaceutical therapies and radioisotope-based treatments for oncology applications in the US market. | Targeted tumor treatment, improved therapeutic outcomes, and minimized exposure to healthy tissue. |
 |
US-focused production of diagnostic radiopharmaceuticals and radioisotope imaging agents for cardiac and PET imaging. | Faster and more accurate disease detection, improved patient management, and strong supply reliability. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The major players in the US nuclear medicine market include GE HealthCare (US), Cardinal Health (US), Curium (France), and Bayer AG (Germany), which actively support the US market with radiopharmaceuticals and radiopharmacy services to provide diagnostic and therapeutic services. These companies are focused on advancing PET/CT and SPECT/CT technologies, theranostics, and the reliable supply of radioisotopes to obtain better clinical outcomes and adherence. US hospitals and imaging centers deploy their solutions widely, and innovations and market expansion efforts are ongoing through consistent collaborations.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
US Nuclear Medicine Market, By Type
Based on type, the US nuclear medicine market is segmented into diagnostic nuclear medicine and therapeutic nuclear medicine, with the diagnostic segment accounting for the largest share. This is because it has been extensively incorporated into routine clinical pathways in US hospitals and outpatient imaging facilities with a firmly developed nuclear pharmacy and reimbursement system. Extensive access to FDA-approved diagnostic radiopharmaceuticals, high throughput of the procedures, and standard clinical guidelines have further enhanced the dominance of diagnostic nuclear medicine in the US healthcare system.
US Nuclear Medicine Market, By Application
Based on application, the diagnostic applications segment holds a significant share in the US nuclear medicine market due to its established role in clinical decision-making and care pathway planning across major disease areas. US healthcare providers are among adopters of the nuclear diagnostics disease staging, therapy choice, and treatment monitoring with the aid of the standardized clinical practices and high coverage of payers. Moreover, there is an availability of FDA-approved diagnostic radiotracers, and the presence of an extensive network of nuclear pharmacy, so the diagnostic nuclear medicine can be easily accessible and used in all hospitals and imaging facilities.
US Nuclear Medicine Market, By Volume Assessment
Based on volume assessment, the diagnostic procedures segment contributed significantly to the US nuclear medicine market due to the high frequency of the routinely conducted nuclear imaging studies in the clinical environment. A large number of hospitals in the US, as well as outpatient imaging centers, frequently perform diagnostic nuclear medicine procedures as a standard medical course, with defined clinical guidelines and reimbursement models. Most healthcare providers predominantly focus on diagnostic procedures which further supports the high volumes of procedures, follow-up imaging, treatment monitoring, and disease assessment, thereby driving the market growth.
US Nuclear Medicine Market, By End User
Based on end users, the US nuclear medicine market is segmented into hospitals, imaging centers, academic & research centers, and other end users, with the hospitals segment holding a significant share. It is because hospitals serve as primary centers for the provision of complete nuclear medicine services, such as advanced diagnostic imaging, radiopharmaceutical treatments, and multidisciplinary care, leading to increased utilization. US hospitals are more likely to have in-house or partnered nuclear pharmacy access, specialized staff, and infrastructure required to support complex and time-sensitive nuclear medicine procedures, driving higher utilization compared to other end-user settings.
US NUCLEAR MEDICINE MARKET: COMPANY EVALUATION MATRIX
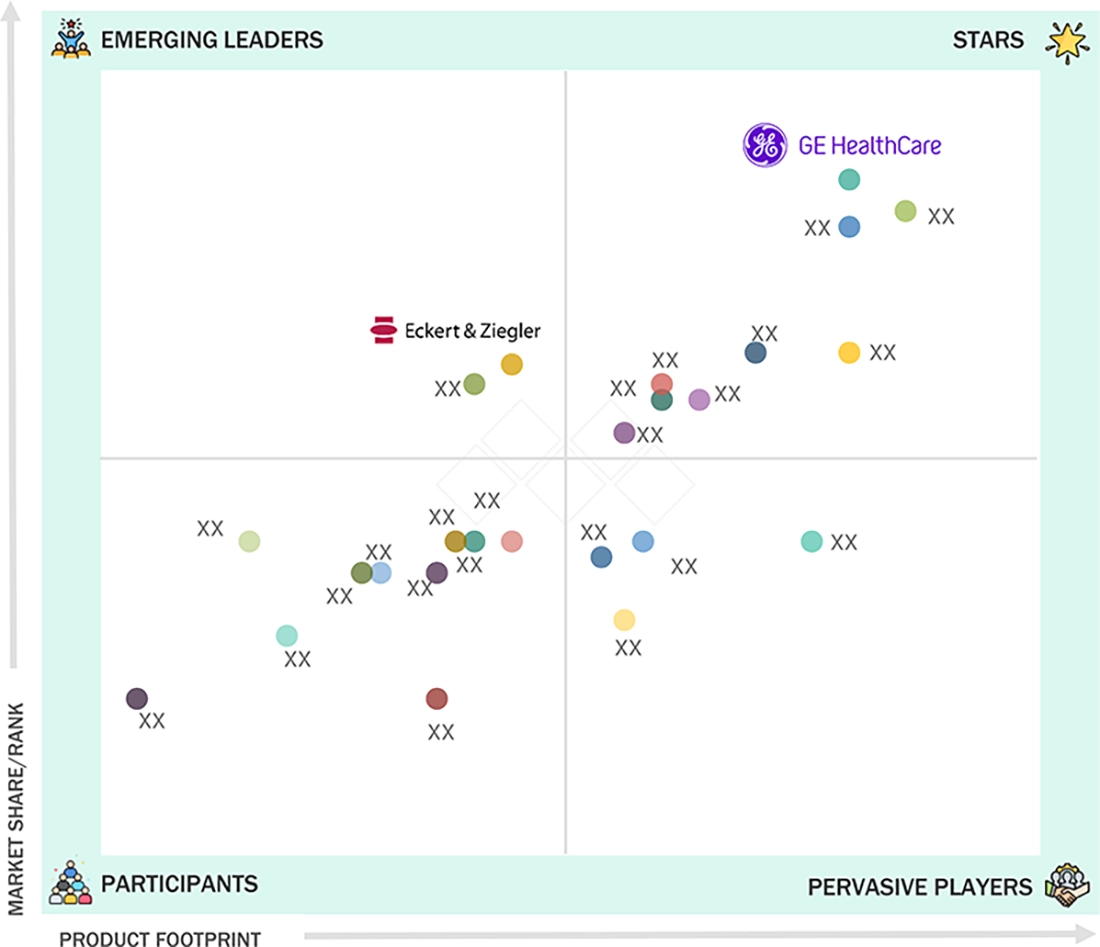
GE HealthCare (Star) is a major player in the US nuclear medicine market, and this is supported by its large nuclear pharmacy network in the US and a wide range of FDA-approved diagnostic and therapeutic radiopharmaceuticals. Eckert & Ziegler (Emerging Leader) has great potential as it is capitalising on its advantages in the production of radioisotopes and radiopharmaceutical development in order to meet the increasing demand of nuclear medicine usage in the US.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- GE HealthCare (US)
- Cardinal Health (US)
- Curium (France)
- Bayer AG (Germany)
- Lantheus Holdings, Inc. (US)
- Novartis AG (Switzerland)
- Jubilant Pharmova Limited (India)
- Bracco Imaging S.P.A (Italy)
- Pharmalogic Holdings Corp. (US)
- Sun Pharmaceutical Industries, Inc. (India)
- Nordion Inc. (Canada)
- Siemens Healthineers AG (Germany)
- NorthStar Medical Radiosiotopes, LLC (US)
- Eckert & Ziegler (Germany)
- Actinium Pharmaceuticals, Inc. (US)
- Global Medical Solutions (US)
- Telix Pharmaceuticals Limited (Australia)
- PDRadiopharma Inc. (Japan)
- ITM Isotope Technologies Munich SE (Germany)
- BWX Technologies Inc. (US)
- SHINE Technologies, LLC (US)
- Isotopia (Israel)
- IRE Elit (Turkey)
- Cyclopharm (Australia)
- RadioMedix, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 4.19 Billion |
| Market Forecast in 2030 (Value) | USD 9.56 Billion |
| Growth Rate | CAGR of 14.7% during 2025-2030 |
| Years Considered | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Country Covered | US |
| Parent & Related Segment Reports |
Nuclear Medicine Market Asia Pacific Nuclear Medicine Market Europe Nuclear Medicine Market |
WHAT IS IN IT FOR YOU: US NUCLEAR MEDICINE MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Competitive Landscape Mapping | The profiles of the major US companies involved in the US nuclear medicine market, such as GE HealthCare, Siemens Healthineers, Curium, Cardinal Health, Lantheus, and Bracco, based on their products and services of radiopharmaceuticals, and imaging systems (PET/SPECT). | Enables competitive benchmarking, differentiation between tracers and imaging modalities, partnership, and investment decision support. |
| Market Entry & Growth Strategy | The US demand analysis in oncology, cardiology, and neurology, the adoption of hospitals and diagnostic centers, reimbursement, and the supply chain of isotopes domestically. | Reduces market entry risks, assists in ranking the indicators of high growth, and supports scalable market expansion strategies. |
| Regulatory & Operational Risk Analysis | US regulatory requirements audit such as FDA approval processes, NRC licenses, radiation safety, and isotope production limitations. | Enhances regulatory preparedness, minimizes the risks of the supply, as well as compliance. |
| Technology Adoption Trends | Understanding the adoption of new radiotracers, theranostics, PET/MR imaging, radiopharmacy automation, and image analysis using artificial intelligence. | Makes product and portfolio strategy information, improves market positioning, and enables concentrated investment in advanced solutions in nuclear medicine solutions. |
RECENT DEVELOPMENTS
- April 2025 : Telix Pharmaceuticals partnered with Cardinal Health to assist with the commercial distribution of its developing gallium-68 PSMA-PET imaging agent Gozellix in the US, enabling wide market availability, following FDA approval.
- March 2025 : GE HealthCare purchased the remaining 50% share of Nihon Medi-Physics Co. Ltd. (NMP) in Sumitomo Chemical, achieving full ownership. NMP focuses on radiopharmaceuticals used in procedures of molecular imaging, such as SPECT and PET, in neurology, cardiology, and oncology.
- October 2024 : Jubilant Radiopharma partnered with Simplified Imaging Solutions to improve efficiencies in operations in nuclear medicine services in the US. This unites their national radiopharmacy system and an organization of diagnostic services to form a single full-service entity.
Table of Contents

Methodology
The study involved four major activities in estimating the current size for the US nuclear medicine market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as D&B Hoovers, Bloomberg Business, and Factiva have been referred to, to identify and collect information for the market study. These secondary sources included annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold standard & silver standard websites, regulatory bodies, and databases.
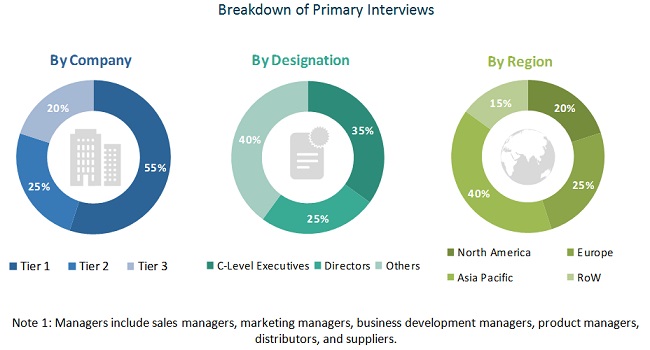
Primary Research
The US nuclear medicine market comprises several stakeholders, such as preferred suppliers and distributors, healthcare institutions (hospitals, medical schools, group practices, individual surgeons, and governing bodies), medical device vendors/service providers, research institutes, and research and consulting firms. Various primary sources from both the supply and demand sides of the market were interviewed to obtain qualitative and quantitative information. Mentioned below is the breakdown of primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the US nuclear medicine market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry and markets have been identified through extensive secondary research
- The industry’s supply chain and market size, in terms of value, have been determined through primary and secondary research processes
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources
Data Triangulation
After arriving at the overall US nuclear medicine market size—using the market size estimation processes as explained above—the market was split into several segments and subsegments. To complete the overall market engineering process and to arrive at the exact statistics of each market segment and subsegment, the data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the nuclear medicine industry
Report Objectives
- To define, describe, and forecast the US nuclear medicine market on the basis of type, application, procedural volume, and country
- To provide detailed information regarding the major factors influencing the growth of the US nuclear medicine market (such as drivers, restraints, and opportunities)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall US nuclear medicine market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders in the US nuclear medicine market
- To forecast the size of the market segments with respect to North America (the US and Canada) in the US nuclear medicine market
- To strategically analyze the market structure and profile key players and their core competencies in the US nuclear medicine market
To track and analyze competitive developments such as product launches, expansions, acquisitions, partnerships, and collaborations in US nuclear medicine market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the US nuclear medicine market report:
Product Analysis
- Product matrix, which gives a detailed comparison of the software portfolios of the top five companies
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the US Nuclear Medicine Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation












Growth opportunities and latent adjacency in US Nuclear Medicine Market