The study involved estimating activities to determine the current size of the autoimmunity diagnostics market. Exhaustive secondary research was done to collect information on the autoimmunity diagnostics industry. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, such as top-down and bottom-up, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were used to estimate the market size of the segments and subsegments of the autoimmunity diagnostics market.
Secondary Research
Various sources were utilized to gather information for this study in the secondary research process. These sources included company annual reports, press releases, and investor presentations; white papers; certified publications; articles by recognized authors; reputable websites; and information from regulatory bodies and databases, such as D&B Hoovers, Bloomberg Business, and Factiva.
Primary Research
In the primary research process, various sources from both the supply & demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess prospects.
The following is a breakdown of the primary respondents:
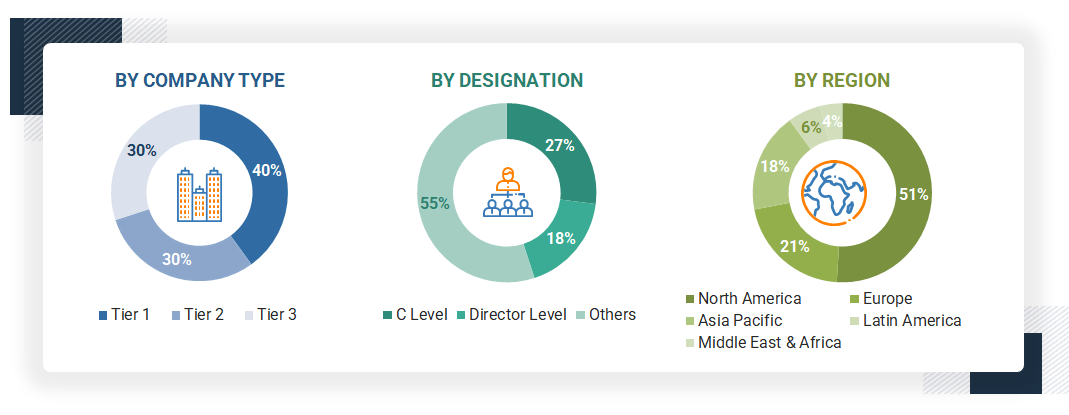
Note 1: Others include sales, marketing, and product managers.
Note 2: Companies are classified into tiers based on their total revenues. As of 2024, Tier 1 = >USD 100 million, Tier 2 = USD 10 million to USD 100 million, and Tier 3 = < USD 10 million.
To know about the assumptions considered for the study, download the pdf brochure
|
COMPANY NAME |
DESIGNATION |
|
Abbott Laboratories (US) |
Product Specialist |
|
Thermo Fisher Scientific Inc. (US) |
Clinical Sales Specialist |
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the autoimmunity diagnostics market's total size. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
-
The key players in the industry were identified through extensive secondary research.
-
Primary and secondary research determined the revenues generated by leading players operating in the autoimmunity diagnostics market.
-
All percentage shares, splits, and breakdowns were determined using secondary sources and verified through primary sources.
The research methodology used to estimate the market size includes the following:
Data Triangulation
The total market was split into several segments after arriving at the overall market size by applying the abovementioned process. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Autoimmune diseases are defined as a medical condition in which an individual's immune system produces antibodies that attack the body's normal tissues. autoimmunity diagnostics systematically identifies autoimmune disorders through clinical assessment and laboratory investigations to detect immune system dysfunction. This process typically involves routine laboratory testing, measurement of systemic inflammatory markers, detection of disease-specific autoantibodies, urinalysis, and other specialized immunologic assays.
Stakeholders
-
Manufacturers & distributors of autoimmunity diagnostics products
-
Clinical laboratories
-
Hospitals
-
Research institutes
-
Government associations
-
Venture capitalists & investors
Report Objectives
-
To define, describe, segment, and forecast the global autoimmunity diagnostics market, by product, test type, disease type, end user, and region
-
To provide detailed information regarding the major factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
-
To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall autoimmunity diagnostics market
-
To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
-
To forecast the size of the market segments with respect to five regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
-
To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
-
To track and analyze company developments such as product launches & approvals, partnerships, acquisitions, agreements, and other developments
-
To benchmark players within the market using the proprietary Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business and product excellence



Growth opportunities and latent adjacency in Autoimmunity Diagnostics Market