
Europe Apheresis Market Size, Growth, Share & Trends Analysis
Europe Apheresis Market by Product (Device (Centrifugation, Membrane Separation) Disposable), Procedure (Donor, Therapeutic), Application [Plasmapheresis, Plateletpheresis, Leukapheresis), Technology (Centrifugation), End User - Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
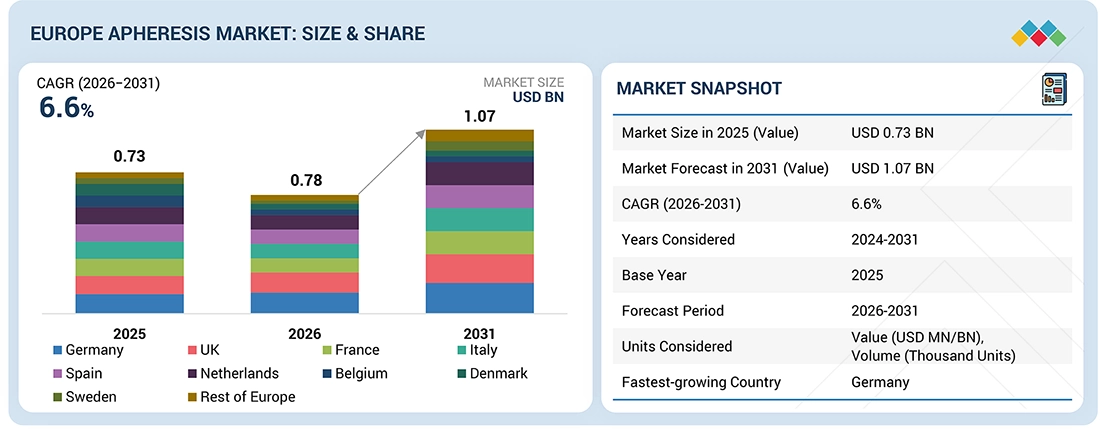
The Europe Apheresis market, valued at USD 0.73 billion in 2025, stood at USD 0.78 billion in 2026 and is projected to advance at a resilient CAGR of 6.6% from 2026 to 2031, culminating in a forecasted valuation of USD 1.07 billion by the end of the period. Growth of the Europe Apheresis market is mainly driven by increased demand for blood components, the presence of strong platelet apheresis infrastructure, widespread adoption of next-generation apheresis devices, and continuous investment in the healthcare ecosystem.
KEY TAKEAWAYS
-
By CountryThe Germany apheresis market accounted for a 27.7% revenue share in 2025.
-
By ProductBy product, the apheresis disposable segment is expected to dominate the market with 80.8% share in 2025.
-
By ProcedureBy procedure, the automated blood collection segment accounts for 75.3% in 2025.
-
By TechnologyBy technology, the continuous flow centrifugation segment is expected to register the largest market share in 2025.
-
By ApplicationBy application, the plasmapheresis is projected to grow at the fastest rate from 2026 to 2031.
-
By End UserBy end user, the hospitals & transfusion centers are the fastest-growing segment in the Europe apheresis market during the forecast period.
-
Competitive LandscapeCompanies such as Fresenius SE & Co. KGaA, B. Braun SE, and Terumo BCT, Inc. were identified as some of the star players in the European market, given their strong market share and product footprint.
-
Competitive LandscapeCompanies such as Medicap Clinic GmbH, LMB Technologies GmbH, and Bioelettronica S.r.l., among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The Europe Apheresis market is growing rapidly owing to the convergence of technological and medical trends. Clinical demand is rising, prompting healthcare facilities to install safer, more automated apheresis devices for cancer care and autoimmune diseases, as well as for the growing demand for plasma and cell-based advanced therapies driven by population aging.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
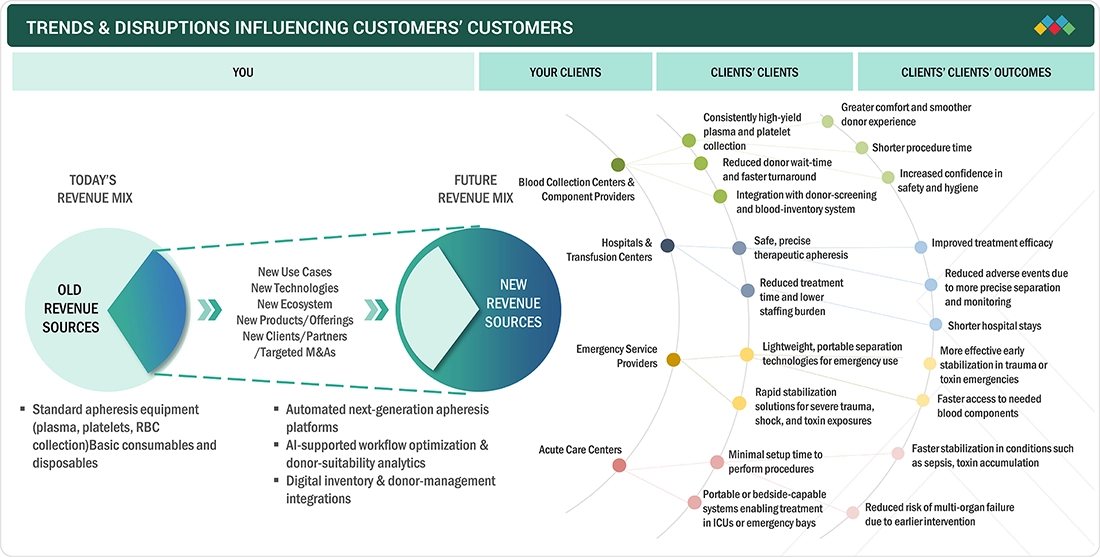
The Europe apheresis market is poised for significant revenue growth over the next 7 to 10 years, primarily driven by changes in the revenue model. New revenue streams will come mainly from the introduction of next-generation apheresis platforms featuring full automation, AI-backed workflow optimization, donor-suitability analytics, and digital integration. Hospitals, Blood Collection Centers, and Acute Care Centers are already adopting these advancements, meeting key imperatives such as high-yield plasma collection, safe therapeutic apheresis, and portable/bedside-capable systems. Ultimately, these technological and operational improvements lead to better client outcomes, including shorter procedure times, improved treatment efficacy, reduced adverse events, and faster patient stabilization.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising prevalence of chronic diseases and trauma/injury cases

-
Increasing demand for source plasma from biopharmaceutical companies
Level
-
High cost of apheresis procedures and installation through rental models
Level
-
Use of apheresis for leukemia and pediatric patients
Level
-
High regulatory compliance cost
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising prevalence of chronic diseases and trauma/injury cases
The Europe apheresis market is primarily influenced by the rising prevalence of chronic diseases, a key trend driven by the region's demographics. The EU is predominantly affected by chronic, non-communicable diseases (NCDs) such as cardiovascular disease and cancer, while autoimmune disorders account for a large share of deaths and the overall disease burden. This trend is further exacerbated by an aging population. More than half of Europeans aged 65 or older already have at least two chronic conditions. Furthermore, the share of the population aged 65 and above is expected to increase significantly by 2050. This demographic shift is driving the increased popularity of advanced therapeutic solutions. Apheresis procedures, which separate pathogenic substances (such as autoantibodies and inflammatory mediators) from the blood, are becoming increasingly important as precise and efficient ways to manage acute phases of chronic illnesses, support cancer care (e.g., cell collection), and treat difficult autoimmune disorders in hospitals and transfusion centers throughout Europe, thereby continually driving market growth throughout the entire forecast period.
Restraint: High cost of apheresis procedures and installation through rental models
One of the main barriers to the apheresis market in Europe is the high cost of apheresis devices, which indirectly affects the market for apheresis treatments by limiting device accessibility and overall demand. Purchasing apheresis devices requires a substantial upfront investment that most very small hospitals or those operating with limited resources cannot afford. Over time, the expenses associated with apheresis devices include not only the purchase price but also operating costs, consumables, and professional training for doctors and nurses. Healthcare professionals will likely consider the financial returns from the procedure; otherwise, the technology will not be profitable, thereby restricting patients' opportunities to receive life-saving treatments. Furthermore, concerns about the cost-effectiveness of apheresis relative to alternative therapies or traditional blood banking methods may also contribute to low adoption rates over the long run.
Opportunity: Use of apheresis for leukemia and pediatric patients
The Europe apheresis market has a strong likelihood of being driven by the region's focus on specialized oncology and the rise of advanced cellular therapies, making it a major growth area, particularly in the treatment of leukemia and pediatric patients. In therapeutic apheresis, leukapheresis plays a critical role, as it is the only way to immediately control hyperleukocytosis in acute leukemia and stabilize patients, especially children, until chemotherapy begins. This growth is being accelerated by the rapid acceptance of Cell and Gene Therapy (CGT), such as CAR T-cell therapy, for children and adults with blood cancers. For this, apheresis is the necessary first step, collecting the patient's own T-cells for reprogramming. More and more treatment centers and clinical trials for CGT are emerging in Europe, together with the creation of specialized, low-volume apheresis protocols and kits for pediatric patients. As a result, demand for therapeutic procedures and advanced apheresis equipment will remain high and sustained across the continent, representing a high-value demand.
Challenge: High regulatory compliance cost
Compliance with the strict traceability requirement (to track each unit from the donor to the recipient) and extensive donor screening and testing (mandatory for both known and new pathogens, such as vector-borne diseases due to climate change) make it possible to use only the most sophisticated testing methods alongside the highest-quality systems. This IMT (invasive medical technology) objectionable scenario caused by the new SoHO Regulation adds cost and complexity to apheresis operations, which surely limits investments in new equipment.
EUROPE APHERESIS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Therapeutic apheresis systems & plasma collection technologies | High-quality plasma collection, efficient therapeutic procedures, strong European presence |
 |
Automated blood component collection & therapeutic apheresis | Advanced automation, improved donor safety, consistent component yield |
 |
Plasma collection devices & donor management software | Enhanced workflow efficiency, reduced collection time, optimized donor management |
 |
Therapeutic apheresis filters & membrane separation technologies | High selectivity, improved patient outcomes, strong reputation in immunological & metabolic disorder care |
 |
Apheresis kits, disposables, and blood component collection bags | High-quality disposables, strong regulatory compliance, customization for European blood centers |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe apheresis market ecosystem is characterized by close collaboration among Manufacturers (providing state-of-the-art automated equipment and disposables), Blood Collection Centers, and Transfusion Services (primary high-volume users for plasma and platelet collection), as well as Specialized Hospitals/Acute Care Centers (handling complex therapeutic apheresis for oncology and autoimmune diseases, and managing patients in Cell & Gene Therapy (CGT)). The ecosystem is tightly regulated under EU Directives and by National Health Authorities, ensuring safety and quality standards so high that they, in turn, drive the adoption of further innovative and compliant technologies. Research Institutions and Biopharma Companies are also integral to this ecosystem, demanding high-quality source plasma and immune cells, thereby sustaining the market's high-value, specialized growth trajectory.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Apheresis Market, By Product
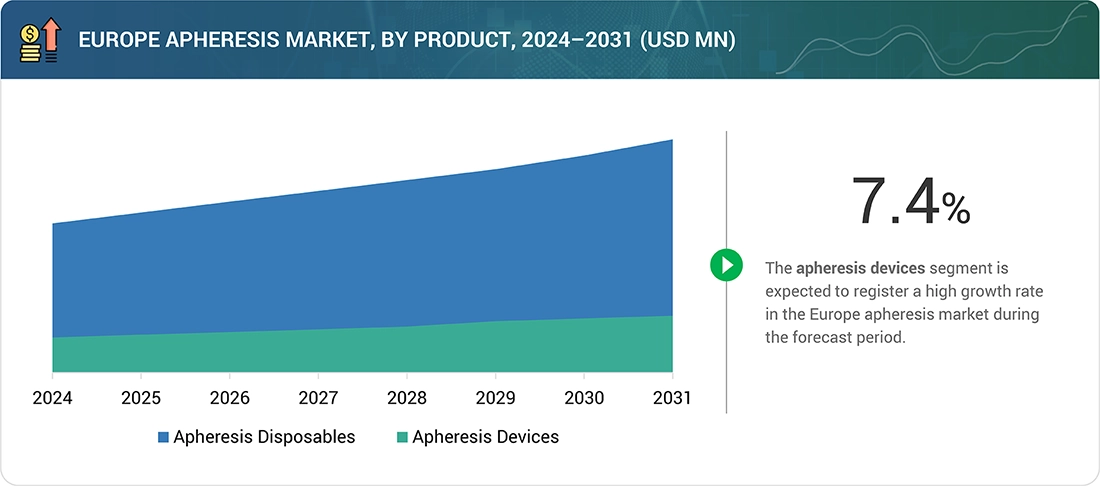
By product, the Europe Apheresis market is segmented into apheresis devices and disposables. Among these, the apheresis device segment is expected to grow at the fastest rate. The apheresis device market is further classified into two primary sub-segments, i.e., centrifugation apheresis devices and membrane separators. The centrifugation apheresis device is expected to dominate the apheresis device segment by occupying the largest share in 2025, owing to its numerous advantages over membrane separation technology. As the most efficient blood component separator, the centrifugation apheresis device has gained maximum popularity among healthcare providers, as it is the most efficient and effective tool for therapeutic procedures. Moreover, these devices are mostly used in the treatment of various conditions, including leukemia, autoimmune disorders, and neurological diseases. Therefore, there is rising demand for centrifugation apheresis devices in hospitals, which is consequently driving the growth of the segment in the apheresis market.
Europe Apheresis Market, By Application
By application, the plasmapheresis segment is the major contributor to the market, as it plays a prominent role in treating a wide range of neurological, immunological, and renal disorders. Plasmapheresis, or therapeutic plasma exchange, is commonly used in the management of complex conditions such as Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), myasthenia gravis, thrombotic thrombocytopenic purpura (TTP), renal diseases, and rheumatic diseases. Its mechanism of action allows the removal of antibodies, immune complexes, and other pathogenic factors from a patient's plasma, thereby meeting the needs of patients with debilitating illnesses and demonstrating the treatment's marked effectiveness. Therefore, with the rising incidence of neurological and autoimmune diseases, demand for plasmapheresis would continue to grow significantly in the competitive and rapidly developing market space.
Europe Apheresis Market, By End user
By end user, blood collection centers and blood component providers segment accounted for the largest share of the Europe Apheresis market. The market potential for blood collection centers and blood component providers is substantial, driven by rising demand for apheresis devices and related disposables. These facilities constitute a key part of the healthcare ecosystem, collecting, processing, and distributing blood products to meet patients' needs for various medical treatments, surgeries, and transfusions. The growing demand for blood components such as plasma, platelets, and red blood cells prompts these facilities to adopt advanced apheresis technologies to efficiently collect and separate these components from whole blood donations. As a result, this rising demand from blood collection centers and blood component providers will enhance market growth during the forecast period.
REGION
UK to be fastest-growing country in Europe Apheresis market during forecast period
Germany is projected to record the highest CAGR over the forecast period. Germany's accelerating growth is driven by factors such as increasing adoption of automated therapeutic apheresis procedures, a close focus on boosting domestic plasma collection for essential plasma-derived therapies, and a strong reimbursement structure supporting the adoption of apheresis procedures.

EUROPE APHERESIS MARKET: COMPANY EVALUATION MATRIX
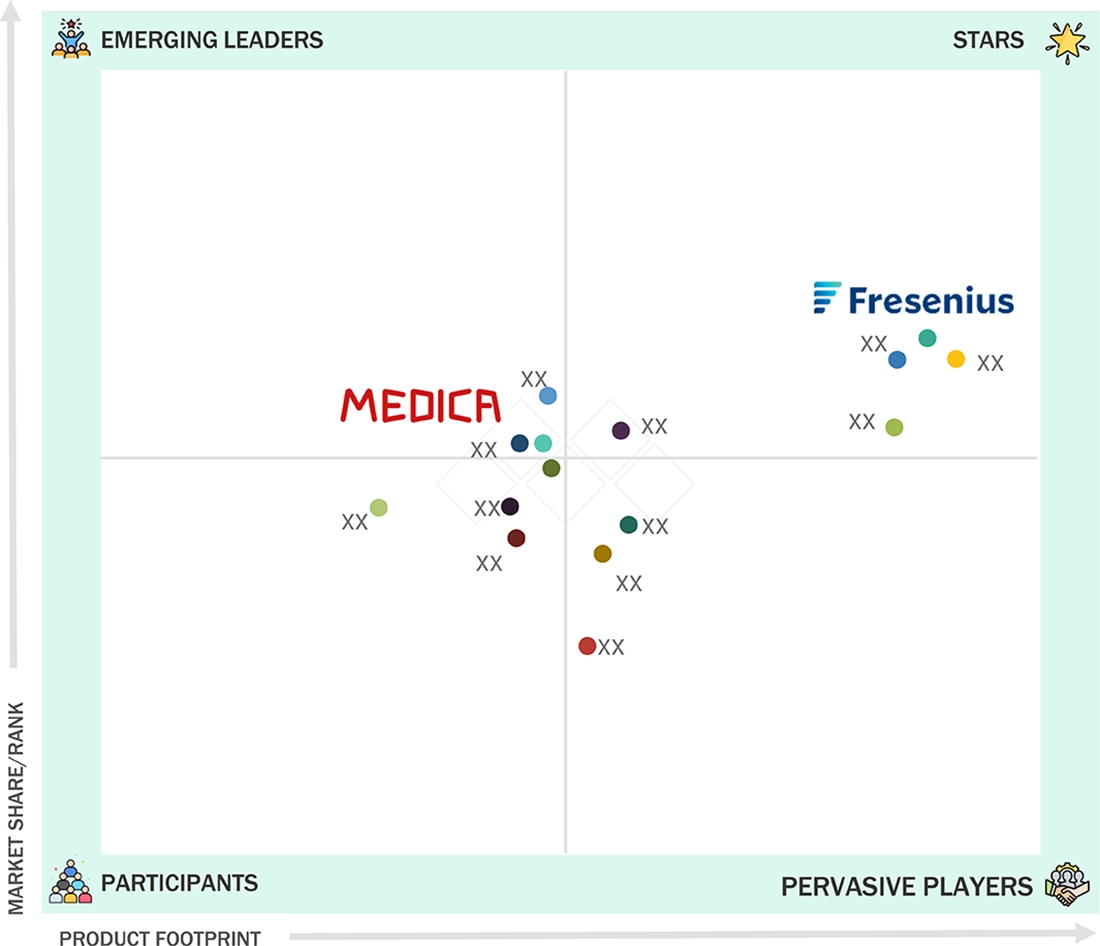
In the Europe Apheresis market matrix, Fresenius Kabi (Star) holds a leading position with a strong market share and an extensive, diverse product footprint, particularly in therapeutic apheresis and essential blood collection disposables. The company's strength is driven by its deep integration into the European healthcare system and its comprehensive portfolio, including sophisticated platforms. Conversely, it is gaining traction with its technical expertise in membrane separation and specialized niche products, such as advanced plasma filters, plasma fractionators, and leukocyte adsorbers, particularly for pediatric and therapeutic applications. While Fresenius Kabi dominates through scale, global presence, and a wider range, Medica S.p.A. shows significant potential to capture a larger share, particularly as demand for advanced membrane-based filtration and targeted extracorporeal treatments continues to expand across European hospitals.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Terumo BCT, Inc. (Japan)
- Fresenius SE & Co. KGaA (Germany)
- Haemonetics Corporation (US)
- Baxter International Inc. (US)
- Asahi Kasei Medical Co., Ltd. (Japan)
- Medica S.p.A. (Italy)
- Becton, Dickinson and Company (US)
- LMB Technologie GmbH (Germany)
- Bioelettronica S.r.l. (Italy)
- Infomed SA (Switzerland)
- Medicap clinic GmbH (Germany)
- B. Braun Melsungen AG (Germany)
- Macopharma SA (France)
- Mallinckrodt plc (Ireland)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 0.73 Billion |
| Market Forecast in 2031 (Value) | USD 1.07 Billion |
| Growth Rate | CAGR of 6.6% from 2026-2031 |
| Years Considered | 2024-2031 |
| Base Year | 2025 |
| Forecast Period | 2026-2031 |
| Units Considered | Value (USD Million/Billion), Volume (Kiloton) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, Italy, France, Spain, UK, Belgium, Sweden, Netherlands, Denmark, Rest Of Europe |
| Parent & Related Segment Reports | Apheresis Market |
WHAT IS IN IT FOR YOU: EUROPE APHERESIS MARKET REPORT CONTENT GUIDE
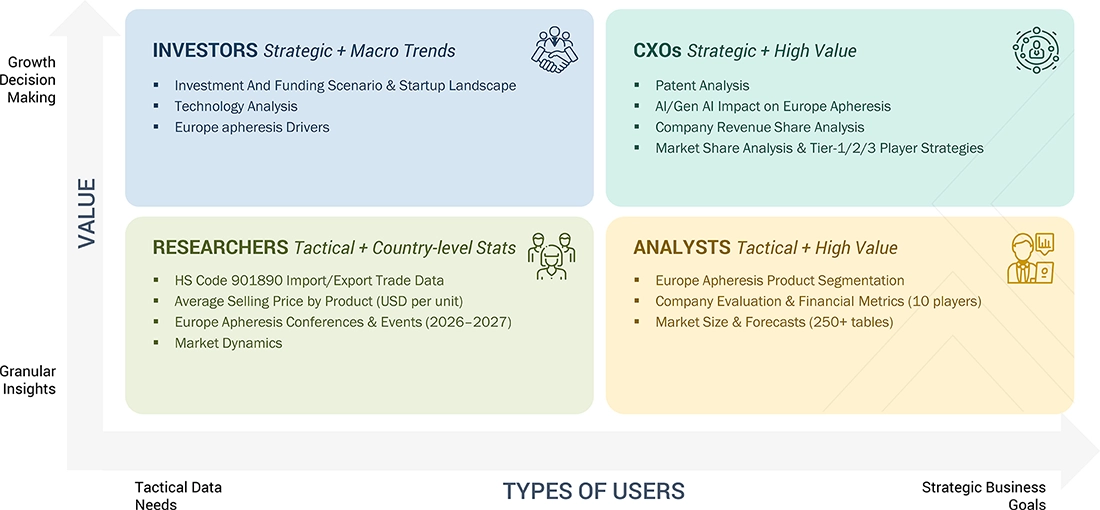
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Detailed assessment of apheresis products by type (e.g., plasmapheresis, leukapheresis, plateletpheresis, erythrocytapheresis, photopheresis). | Analysis of emerging trends such as growing use of therapeutic apheresis in autoimmune disorders, oncology, and neurology, adoption of next-generation automated platforms, and expanded plasma collection to support biopharma manufacturing. |
| Company Information | Comprehensive profiles of major players such as Fresenius Kabi, Terumo BCT, Haemonetics, Asahi Kasei, Macopharma, and key European regional manufacturers. | Identification of strategic collaborations, government-supported plasma initiatives, R&D partnerships, and M&A activities shaping the Europe apheresis landscape. |
| Geographic Analysis | Country-level demand mapping across Germany, France, U.K., Italy, Spain, Netherlands, and Eastern Europe. | Country-specific market outlook highlighting growth opportunities, regulatory updates, reimbursement dynamics, and technology adoption patterns across major European markets. |
RECENT DEVELOPMENTS
- August 2023 : Fresenius Kabi and Lupagen forged a strategic development and supply agreement aimed at advancing cell and gene therapies for clinical application
- June 2023 : Haemonetics obtained FDA clearance for upgrades to its NexSys PCS Plasma Collection System, signaling advancements in the efficiency and functionality of the platform
- May 2022 : Terumo BCT Inc. opened its second manufacturing facility in Colorado.
- December 2022 : Fresenius Kabi expanded its critical care portfolio in the US, this will help increase the market growth for apheresis.
Table of Contents

Methodology
The study involved four major activities to estimate the current size of the Europe Apheresis market. Exhaustive secondary research was done to collect information on the market and its different subsegments. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. Thereafter, market breakdown and data triangulation procedures were used to estimate the market size of the segments and subsegments.
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to in order to identify and collect information for the study of Europe Apheresis Market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
Extensive primary research was conducted after obtaining information regarding the Europe Apheresis market scenario through secondary research. Several primary interviews were conducted with market experts from both the demand and supply sides across Europe. Primary data was collected through questionnaires, emails, and telephonic interviews. The primary sources from the supply side included various industry experts, such as Chief X Officers (CXOs), Vice Presidents (VPs), Directors from business development, marketing, product development/innovation teams, and related key executives from manufacturers; distributors operating in the Europe Apheresis market.; and key opinion leaders.
Primary interviews were conducted to gather insights such as market statistics, data on revenue collected from the products and services, market breakdowns, market size estimations, market forecasting, and data triangulation. Primary research also helped in understanding the various trends related to technology, application, vertical, and region. Stakeholders from the demand side customers/end users who are using infection control products were interviewed to understand the buyer’s perspective on the suppliers, products, and their current usage and the future outlook of their business, which will affect the overall market.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The research methodology used to estimate the size of the Europe Apheresis market includes the following details.
The market sizing of the market was undertaken from the global side.
Country-level Analysis: The size of the Europe Apheresis market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products in the overall Europe Apheresis market was obtained from secondary data and validated by primary participants to arrive at the total Europe Apheresis market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by end users and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall Europe Apheresis market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes—the market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and sub-segment, the data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides in the europe apheresis industry.
Market Definition:
The Apheresis market encompasses the global business sphere surrounding the development, production, distribution, and use of apheresis equipment and related consumables. This specialized technology enables the separation and collection of specific blood components, such as plasma, platelets, or white blood cells, from a whole blood donation. These collected components are then used in various therapeutic applications, including treating blood disorders, autoimmune diseases, and neurological conditions.
Key Stakeholders
- Europe Apheresis devices manufacturers and distributors
- Hospitals and transfusion centers
- Therapeutic europe apheresis service providers
- Blood banks and blood collection centers
- Plasma fractionation companies
- Healthcare providers
- Academic research institutes
- Pharmaceutical companies
- Contract research organizations (CROs)
- Market research & consulting firms
Report Objectives
- To define, describe, segment, and forecast the Europe Apheresis market by product, technology, procedure, application, end user, and region.
- To provide detailed information about the factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall Europe Apheresis market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players.
- To profile key players in the Europe Apheresis market and comprehensively analyze their core competencies and market shares.
- To track and analyze competitive developments such as acquisitions, expansions, partnerships, agreements, and collaborations; and product launches and approvals.
- To benchmark players within the Europe Apheresis market using the "Company Evaluation Matrix" framework, which analyzes market players on various parameters within the broad categories of business and product strategy.
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
- Product Analysis: Product matrix, which gives a detailed comparison of the product portfolios of each company
- Geographic Analysis: Further breakdown of the Latin America apheresis imarket nto specific countries, the Middle East and Africa Apheresis market into specific countries, and the Europe apheresis into specific countries
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Apheresis Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Europe Apheresis Market