
Europe Sleep Apnea Devices Market Size, Growth, Share & Trends Analysis
Europe Sleep Apnea Devices Market by Product [Therapeutic {PAP (CPAP, APAP, BPAP), Oral Appliances, Masks}, Diagnostics {PSG, Home Sleep Testing, Oximeter}]; Age, Gender, End User [Sleep Clinics, Hospitals, Home Care Settings] - Forecast to 2031




OVERVIEW
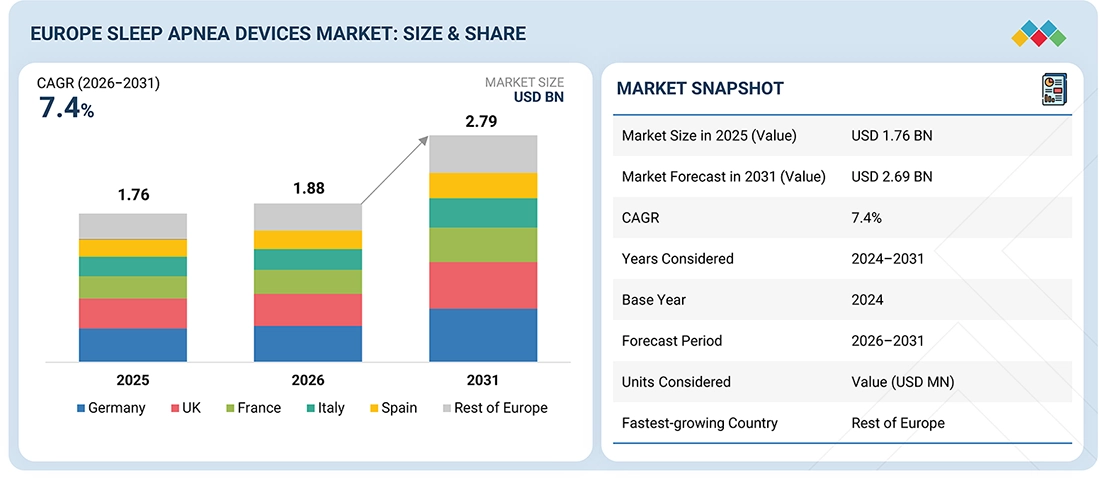
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The Europe Sleep Apnea Devices market, valued at US$1.76 billion in 2025, stood at US$1.88 billion in 2026 and is projected to advance at a resilient CAGR of 7.4% from 2026 to 2031, culminating in a forecasted valuation of US$2.79 billion by the end of the period. Market growth across Europe is driven primarily by the rising prevalence of obstructive sleep apnea and other chronic conditions, such as obesity and CVD. Rising awareness of the serious health risks associated with untreated sleep apnea also contributes to demand for timely diagnosis and treatment. Furthermore, accelerating demand for reliable diagnostic and therapeutic solutions is driving the pace of sleep apnea device adoption across the region. Recent healthcare infrastructure development, increasing insurance coverage in many European nations, and greater adoption of home-based sleep testing and PAP therapy devices are driving growth significantly. Additionally, the growing focus on early detection, patient-centered care models, and continuous technological advancements in sleep apnea devices should ensure strong growth in the European market in the next few years.
KEY TAKEAWAYS
-
By ProductBy product, the therapeutic devices segment is expected to register the highest CAGR of 7.5%.
-
By End UserBy end user, the home care settings & self-testing is expected to be the fastest-growing segment.
-
By Age GroupBy age group, the 40-60 years segment accounted for 57.5% of the market in 2025.
-
By Sample TypeBy gender, the male segment accounted for the largest share of the market in 2025.
-
Competitive Landscape - Key PlayersResMed, Koninklijke Philips N.V., Fisher & Paykel Healthcare Limited, Inspire Medical Systems, Inc., and SomnoMed were identified as as star players in the Europe sleep apnea market, supported by their strong market presence and extensive product portfolios.
-
Competitive Landscape - Startups/SMEsOpenairway, Compumedics Limited, Löwenstein Medical SE & Co. KG, Drive DeVilbiss International, BMC, and BRAEBON Medical Corporation, and among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas.
The key factors driving growth in the sleep apnea devices market in Europe include the rising prevalence of obstructive sleep apnea, increased awareness of sleep-related risks, and growing demand for home sleep testing and positive pressure therapy devices. Supportive reimbursement policies, regulatory environments, and access to specialized sleep care services are also important contributors to the growth of the sleep apnea devices segment. In recent times, technology integration, design advantages offered by these devices, and the development of healthcare infrastructure, especially in Western and Central European nations, have contributed significantly to the growth and expansion of the sleep apnea devices segment.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The sleep apnea devices market in Europe is experiencing continuous growth, driven by the region's strong emphasis on rapid diagnosis, timely results, and structured management of sleep-related breathing disorders. The broader integration of diagnostic and therapeutic sleep apnea devices into hospitals, specialized sleep laboratories, pulmonology clinics, and expanding home care services is an emerging trend across the continent. This integrated care approach is believed to enable faster diagnosis and, consequently, earlier initiation of therapy, creating significant growth opportunities for market participants. The rising adoption of solutions such as HST, digitally enabled PAP devices, and remote patient monitoring platforms is significantly affecting the diagnosis and management of sleep apnea across European nations. Moreover, the development of compact, lightweight, and connected devices that improve portability and ease of use is further increasing their utilization in home-based care settings and outpatient facilities. These technological and structural changes in healthcare are collectively strengthening the growth trajectory of the Europe sleep apnea devices market.
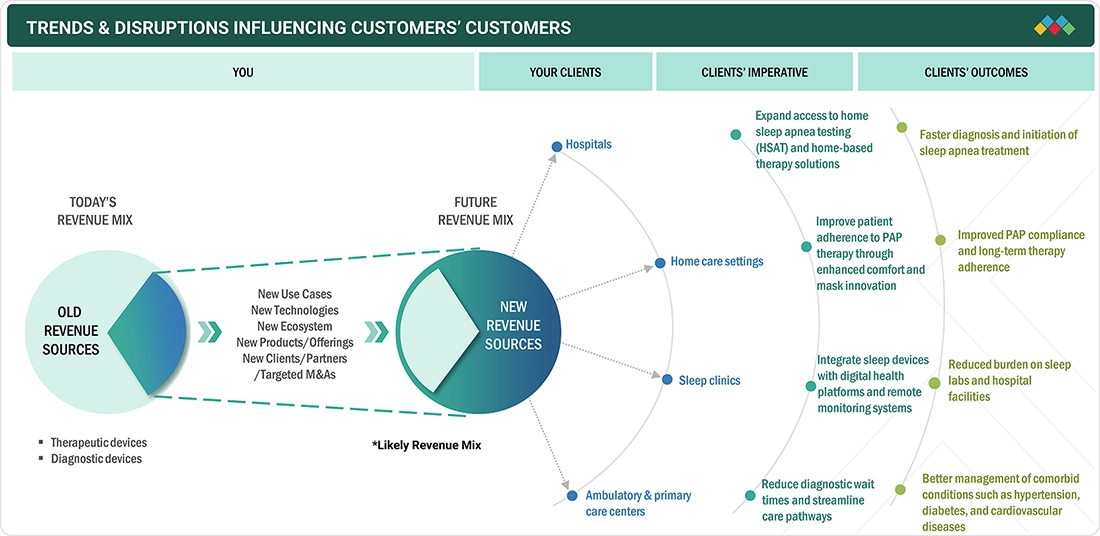
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Favorable reimbursement and financial aid for sleep apnea devices and therapies

-
Large pool of undiagnosed obstructive sleep apnea (OSA) patients
Level
-
Complex referral pathways, long waiting periods, and delayed diagnosis
-
High cost of CPAP devices and minimal insurance access in emerging economies
Level
-
Rising demand for cost-effective home sleep apnea tests
-
Increasing focus on telemedicine, mHealth, and AI
Level
-
Poor patient compliance with CPAP therapy
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Favorable reimbursement and financial aid for sleep apnea devices and therapies
The Europe sleep apnea devices market is undergoing rapid development due to increased investments made by prominent industry players and their strong focus on innovation, especially with CPAP systems, masks, and devices used to diagnose sleep apnea. Market growth is also driven by an improvement in patient comfort and an increase in therapy compliance, with light, quiet, and ergonomic equipment being used to improve these, alongside the reimbursement environment and patient awareness, which enable the proper management and diagnosis of the condition through various innovative therapeutic and diagnostic tools made available to the healthcare industry in Europe.
Restraint: Complex referral pathways, long waiting periods, and delayed diagnosis
One of the major challenges associated with the development and growth of the sleep apnea devices market in Europe is the lack of understanding and underdiagnosis of sleep apnea. A major portion of the population remains unaware that they are clinically experiencing sleep apnea. They are also unable to recognize the common signs and associated symptoms of sleep apnea, such as snoring, excessive sleepiness, headaches, and difficulty concentrating. Awareness is also needed regarding the severe health risks generally associated with clinical sleep apnea, such as cardiovascular problems, hypertension, stroke, diabetes, and metabolic problems.
Opportunity: Rising demand for cost-effective home sleep apnea tests
The Europe sleep apnea devices market has been witnessing a significant shift in the diagnosis and therapy delivered at home. The primary driver is customer demand for convenient, comfortable, and technologically advanced care options. This shift has had a significant impact on home healthcare services, with more people opting for PAP therapy and home sleep apnea testing. Moreover, advancements in next-generation sleep monitoring technology, including connectivity features, smartphone integration, cloud services, and remote monitoring, have further boosted the Europe sleep apnea devices market, as they not only ensure a better customer experience but also increase the accuracy and efficiency of diagnosis and therapy. In addition, recent advancements in wearable technology have significantly enhanced the customer experience during therapy, making home diagnosis more convenient. Thus, home sleep apnea diagnosis is expected to generate significant growth within the Europe sleep apnea devices market.
Challenge: Poor patient compliance with CPAP therapy
In the Europe sleep apnea devices market, one of the most important issues for patients undergoing continuous positive airway pressure (CPAP) treatment is patient compliance with the healthcare organization. Studies on patient compliance have shown that low compliance is common among patients with sleep disorders in Europe. Therefore, it is essential for healthcare providers in Europe to educate patients about the importance of CPAP treatment. By providing more education about CPAP treatment, patient compliance is expected to increase, thereby helping patients receive effective treatment from the healthcare organization.
EUROPE SLEEP APNEA DEVICES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced Positive Airway Pressure (PAP) Therapy Products, cloud-enabled CPAP Therapy Devices, CPAP Therapy Masks, and Home Sleep Apnea Testing Products were shipped to hospitals and homes across Europe. | Ensures improved patient compliance with treatment and comfort | Enables the remote management of patient health conditions via telemonitoring and connected health tools | Helps optimize long-term patient treatment outcomes |
 |
The availability of accessories such as humidified PAP systems, novel interfaces for masks, and respiration support systems was needed to control apnea. | Enhances comfort through sophisticated humidification technology, improves compliance rates, and effectively enables long-term therapy sessions |
 |
Sleep apnea diagnostic and therapeutic products, including CPAP therapy, BiPAP therapy, sleep apnea masks, and monitors, available as part of European healthcare systems | Assist early diagnosis and management of therapy, as well as the user experience through interconnected care |
 |
Provides precision-engineered oral appliance therapy devices for obstructive sleep apnea in dental and specialty clinics across Europe | Offers a line of noninvasive treatment alternatives to CPAP, comforts patients, and improves therapy compliance in CPAP-intolerant patients |
 |
Manufactures and distributes customized oral devices for treating mild to moderate obstructive sleep apnea via dental networks in Europe | Expands access to alternative sleep apnea treatments, increases patient ease of use, and enhances compliance through specially designed products |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe sleep apnea devices market has a number of participants actively involved in the value chain for the products involved. This varies from manufacturers involved in the production and development of sleep apnea treatment devices such as CPAP devices, BiPAP devices, APAP devices, oral medical devices, masks, and home sleep apnea testing devices to component manufacturers, distributors, DME manufacturers, and R&D contributors. Additionally, organizations such as sleep laboratories, hospitals, sleep apnea treatment centers, dental centers, and home healthcare institutions play a significant role in fueling the growth of this market in Europe. In recent years, home care centers and users have emerged as contributors to the value chain for sleep apnea devices in the European region.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
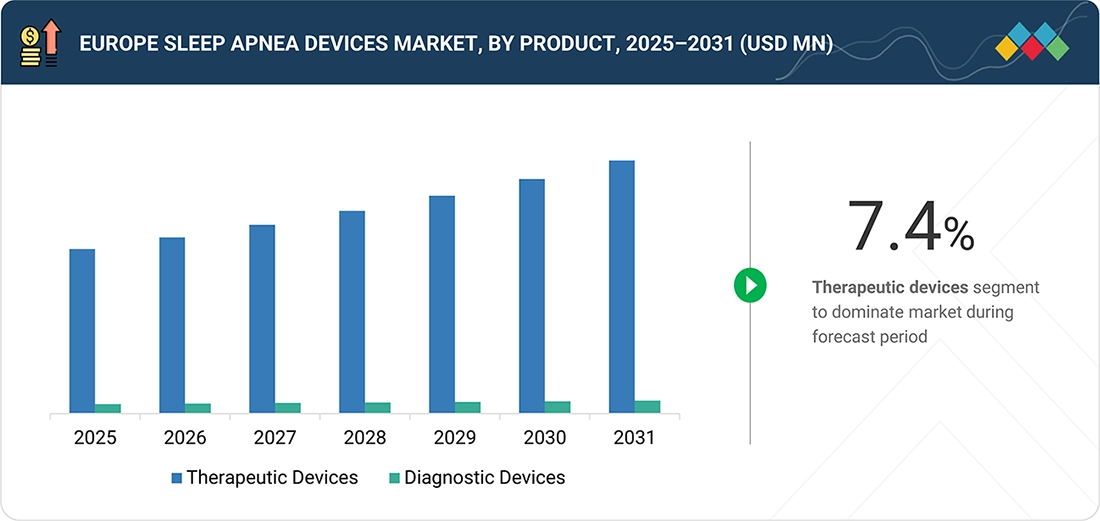
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Sleep Apnea Devices Market, By Product
The therapeutic devices segment is expected to grow at the highest rate in the European sleep apnea market, primarily due to improved diagnosis rates for the disease across the region, coupled with increased acceptance of treatment for the condition. Positive airway pressure (PAP) devices, including continuous positive airway pressure (CPAP), auto-positive airway pressure (APAP), and bilevel positive airway pressure (BiPAP), along with their associated masks, humidifiers, tubes, oral devices, and advanced devices for hypoglossal nerve stimulation, are included in the therapeutic devices segment. Rising awareness of the condition, coupled with home sleep apnea testing and technology innovations geared toward enhancing overall patient comfort, is primarily responsible for the growth of this segment in the European sleep apnea devices market.
Europe Sleep Apnea Devices Market, By End User
The European sleep apnea devices market will witness the fastest growth rate in the home care settings segment. The rising acceptance of home-based diagnosis and treatment of sleep apnea is revolutionizing the way obstructive sleep apnea is treated in Europe. The prevalence of a large number of home sleep apnea testing devices, along with the accessibility of positive airway pressure therapies, has made it more convenient, comfortable, and cost-effective for patients to receive diagnosis and treatment of the disease within the comfort of their own homes. Moreover, the ability of healthcare professionals to track patient compliance with therapy through telemedicine solutions has been contributing significantly to the utilization of home care therapy for the treatment of the disease; this is likely to be very advantageous for the growth of the sleep apnea devices market.
Europe Sleep Apnea Devices Market, By Age Group
In the European market for sleep apnea devices, the 40-60 age group is the largest share of the market. The main reason is the high prevalence of obstructive sleep apnea in this group. People in this age group are more likely to undergo regular health checkups, which increases the detection rate of sleep apnea in this population compared with younger age groups. The strong need for therapeutic interventions, including Positive Air Pressure, Oral Appliances, and other home therapies, is another major factor that helps establish this age group as the biggest market share holder of the sleep apnea market in Europe. Health awareness among this age group is also quite high.
Europe Sleep Apnea Devices Market, By Gender
The male segment is likely to hold a dominant share in the European sleep apnea devices market. The main reason for this is the higher incidence of sleep apnea among men than among women, owing to various physiological factors. The incidence of various sleep disorders is found to be high among men, thereby increasing product awareness among this demographic and resulting in high demand for sleep apnea devices, including PAP devices, masks, and oral appliances. The dominant share held by the male segment is attributed to rising awareness among people of various health hazards and cardiovascular-related health complications resulting from sleep apnea, thereby making them seek sleep apnea devices as a medical intervention.
REGION
Germany is the fastest growing segment by country.
Germany will witness the highest growth rate in the sleep apnea device market in Europe due to growing adoption of home-based solutions and significant investment in the country's healthcare infrastructure. Greater availability of advanced diagnostic technologies, increased focus on early detection of sleep disorders, and unmet needs for decentralized, patient-centric devices and treatments-for diagnostics, therapy, and monitoring of the disease-are driving growth in the country.
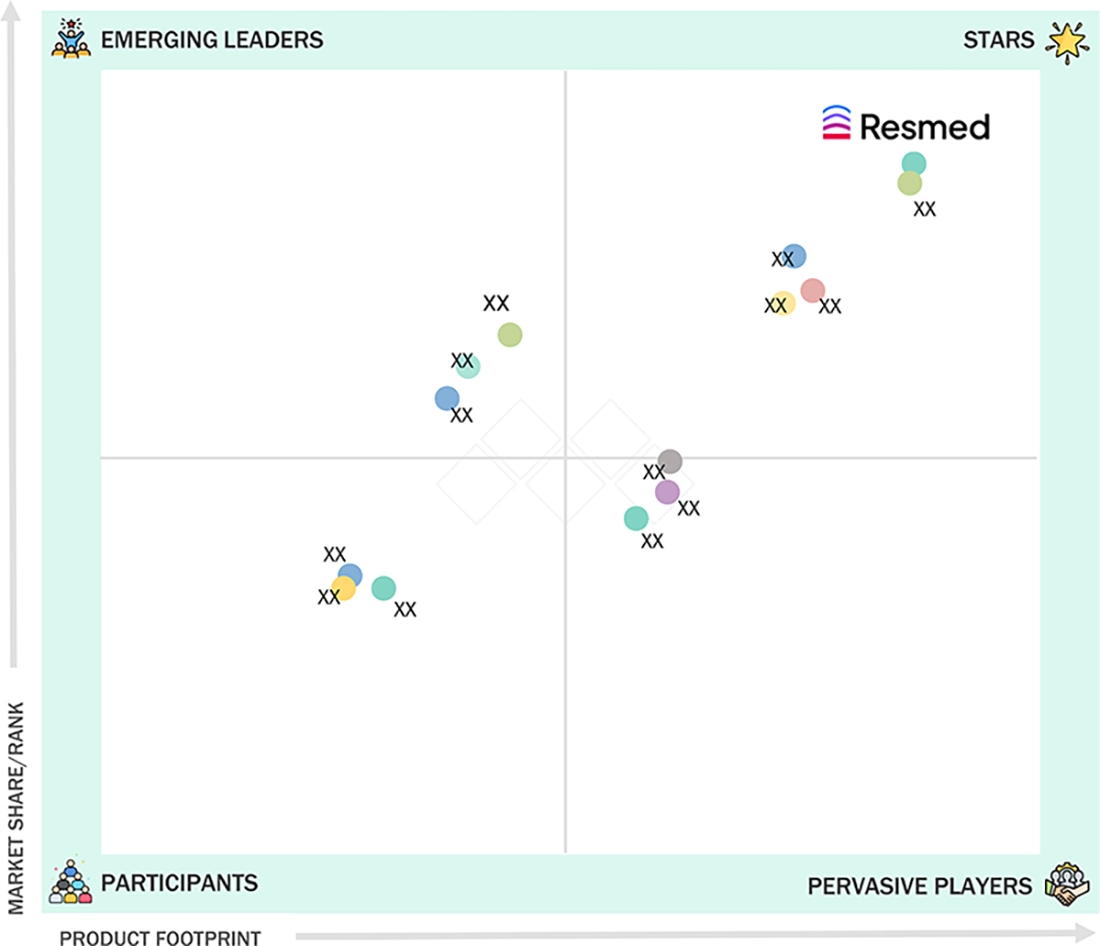
EUROPE SLEEP APNEA DEVICES MARKET: COMPANY EVALUATION MATRIX
ResMed is a well-established company in the Europe sleep apnea devices market, driven by its wide portfolio of innovative therapeutic and diagnostic products in the field of sleep. ResMed has developed a wide range of innovative positive airway pressure (PAP) devices and solutions, including masks, digital health solutions, and cloud-based patient management tools. It also offers a wide range of accessories for its products, which is likely to help the company grow its recurring revenue model. ResMed is a major player through its focus on clinical efficacy, therapeutic innovation, and regulatory compliance, making it a market leader across various segments of the Europe sleep apnea devices market.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 1.76 Billion |
| Market Forecast in 2031 (Value) | USD 2.69 Billion |
| Growth Rate | CAGR of 7.4% from 2026-2031 |
| Years Considered | 2024-2031 |
| Base Year | 2024 |
| Forecast Period | 2026-2031 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, UK, France, Italy, Spain, Rest of Europe |
WHAT IS IN IT FOR YOU: EUROPE SLEEP APNEA DEVICES MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Product matrix, which provides a detailed comparison of the product portfolio of each company in the Europe Sleep Apnea Devices market | Enables easy comparison of competitors’ offerings, helping identify gaps, overlaps, and differentiation opportunities. |
| Company Information | Additional five company profiles of players operating in the Europe Sleep Apnea Devices market | Provides insights into competitors’ strategies, innovation focus, and partnerships, supporting strategic planning. |
RECENT DEVELOPMENTS
- April 2024 : New Zealand-based Fisher & Paykel Healthcare Limited launched the F&P Solo Mask with AutoFit in Europe, available in both nasal and pillow mask formats. The new mask has been designed to make setup easy for both patients and healthcare practitioners. The launch underscores the company’s commitment to the European market for sleep apnea devices, with a focus on ease of use, patient comfort, and adherence to positive airway pressure (PAP) treatment.
- May 2023 : ResMed strengthened its presence in the European sleep apnea device market by acquiring Somnoware, a leader in diagnostic software for sleep and respiratory care. The acquisition expanded ResMed’s portfolio of cloud-based diagnostic and data management solutions, supporting its strategy to deliver technology-enabled solutions that improve efficiency in sleep clinics and home care as part of the broader sleep therapy continuum.
Table of Contents

Methodology
The study analyzes key market dynamics, such as drivers, opportunities, restraints, challenges, and key player strategies. To track company developments such as acquisitions, product launches and approvals, expansions, agreements, and partnerships of the leading players, the competitive landscape of the Europe Sleep Apnea Devices Market is analyzed to evaluate market players on various parameters within the broad categories of business and product strategy. Top-down and bottom-up approaches were used to estimate the market size. The market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
The four steps involved in estimating the market size are
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to identify and collect information for this study.
Primary Research
In the primary research process, various supply and demand sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources were mainly industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess prospects.
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
| COMPANY NAME | DESIGNATION |
|---|---|
| SOMNOmedcis AG (Germany) | Country Manager |
| ResMed (US) | Regional Sales Manager |
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of sleep apnea devices in the market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- The key players in the industry have been identified through extensive secondary research
- Primary and secondary research has determined the revenues generated by leading players operating in the Europe Sleep Apnea Devices Market.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Data Triangulation
After arriving at the overall market size by applying the abovementioned process, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Sleep apnea is a prevalent sleep disorder that is frequently undiagnosed, yet it poses significant health risks. This condition is characterized by intermittent cessation of breathing, which can last from several seconds to minutes, or by irregular breathing patterns. The most predominant form is obstructive sleep apnea (OSA), where anatomical or physiological obstructions impede airflow during sleep. Continuous positive airway pressure (CPAP) devices are commonly used to deliver air at elevated pressure through the nasal passages, effectively counteracting upper airway obstructions and facilitating normal respiration. These interventions are crucial for mitigating the associated morbidities linked with untreated sleep apnea.
Stakeholders
- Senior Management
- Sleep Laboratories, Clinics, & Hospitals
- Home Care Settings
- Academic & research institutes
- Finance/Procurement Department
- R&D Department
Report Objectives
- To define, describe, segment, and forecast the Europe Sleep Apnea Devices Market by product, age group, gender, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, challenges, and trends)
- To analyze the micromarkets with respect to individual growth trends, prospects, and contributions to the overall Europe Sleep Apnea Devices Market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the market segments with respect to five regions—North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa.
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To benchmark players within the market using the proprietary Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business and product excellence
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Sleep Apnea Devices Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Europe Sleep Apnea Devices Market