
Latin America Molecular Diagnostics Market Size, Growth, Share & Trends Analysis
Latin America Molecular Diagnostics Market by Product & Service (Kits, Instruments), Test Type (Lab, PoC), Sample (Blood, Urine), Technology (PCR, NGS, ISH), Application [Infectious (Hepatitis, HIV, HAI, Flu), Cancer (Breast, Lung)] - Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
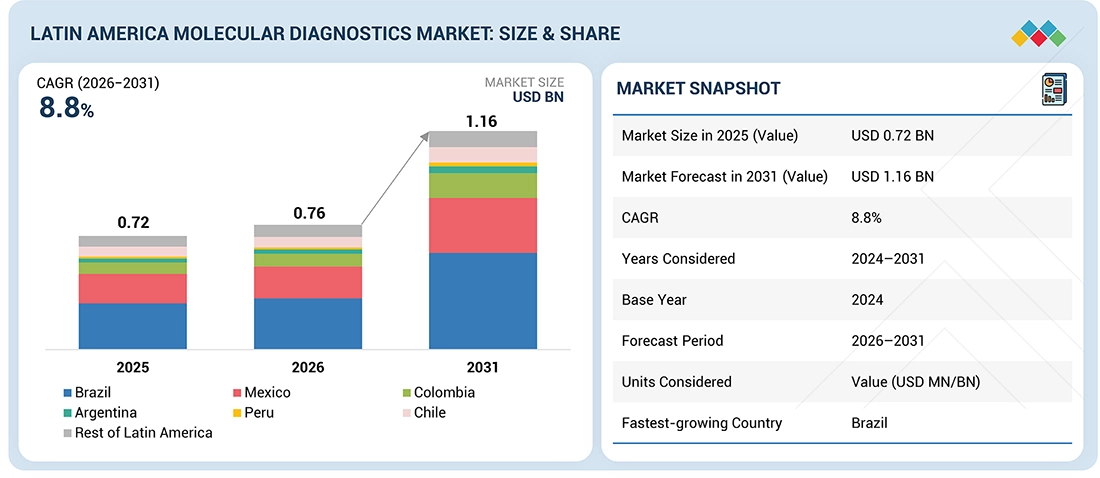
The Latin America molecular diagnostics market, valued at US$0.72 billion in 2025, stood at US$0.76 billion in 2026 and is projected to advance at a resilient CAGR of 8.8% from 2026 to 2031, culminating in a forecasted valuation of US$1.16 billion by the end of the period. This growth can be attributed to the rising demand for reliable diagnostic results, especially in infectious diseases & chronic illnesses like cancer. Improvements in healthcare infrastructure & accessibility of diagnostic services and awareness of early diagnostic testing are some factors that are supporting its greater uptake. Additionally, an increased role of private players & government policies are ensuring consistent growth of the market.
KEY TAKEAWAYS
-
By CountryBy country, Brazil held the largest share of the market in 2025.
-
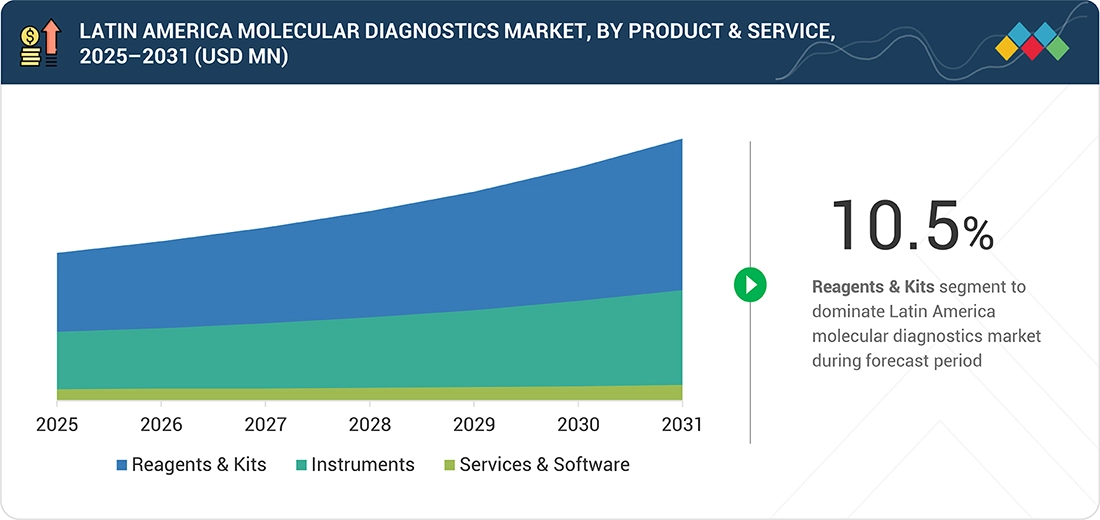
By Product & ServicesBy product & services; the reagents & kits segment is expected to register the highest CAGR of 10.5%.
-
By Test TypeBy test type, the lab tests segment accounted for the largest market share of 75.2% in 2025.
-
By Sample TypeBy sample type, the blood, serum, and plasma segment accounted for the largest market share of 64.4% in 2025.
-
By TechnologyBy technology, the PCR segment accounted for the largest share of the market in 2025.
-
By ApplicationBy application, the infectious disease diagnostics segment is expected to dominate the market.
-
By TechniqueBy technique, multiplex testing accounted for the largest market share in 2025.
-
Clinical ApplicationBy clinical application, the diagnostics segment is expected to dominate the market.
-
By End UserBy end user, the diagnostic labs segment is expected to register the highest CAGR.
-
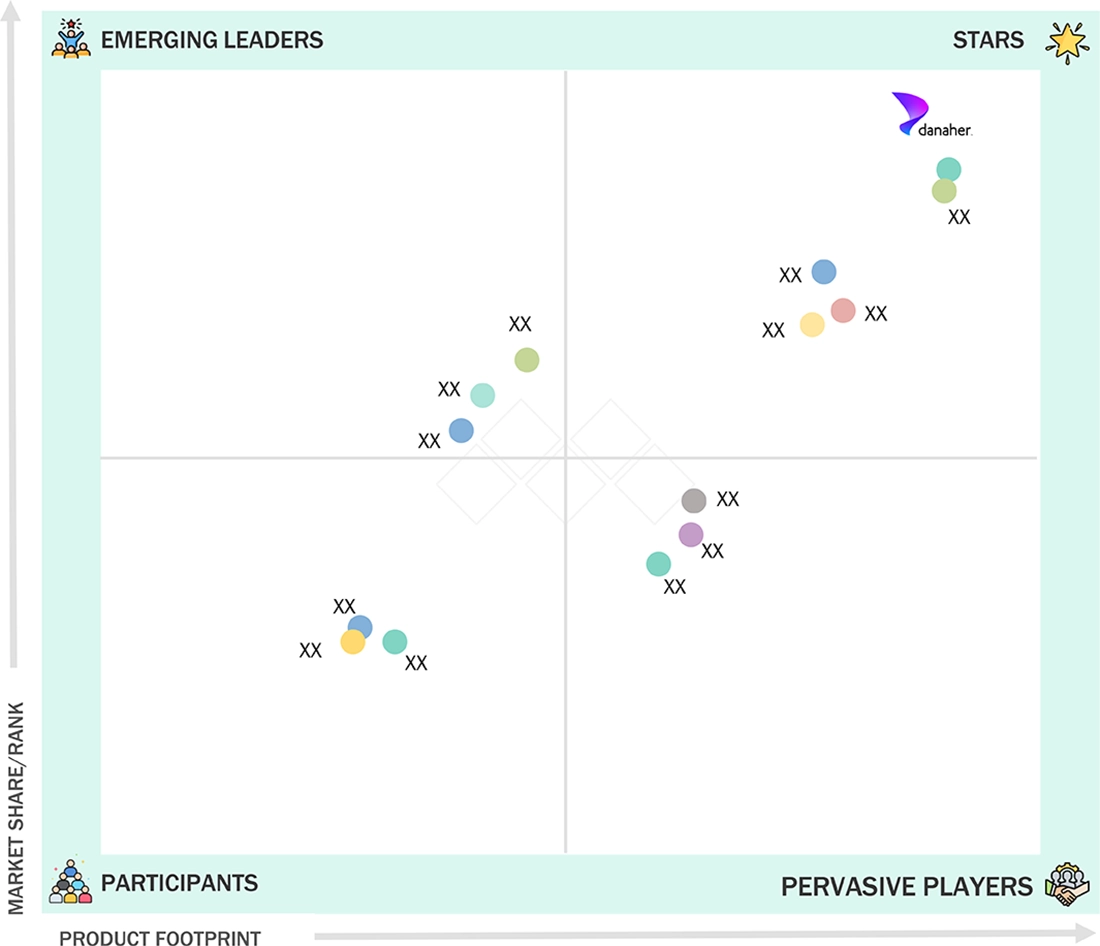
Competitive LandscapeDanaher (US), Roche (Switzerland), Illumina (US), bioMérieux (France), and Thermo Fisher Scientific (US) were identified as star players in the market, supported by their strong market presence and extensive product portfolios.
-
Competitive LandscapeVela Diagnostics (Singapore), Savyon Diagnostics (Israel), and Uniogen OY (Finland) have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas.
The Latin America molecular diagnostics market is driven by the increasing role of molecular testing in routine disease management. Healthcare providers are increasingly using molecular diagnostics to enhance the accuracy of infection identification and support better informed decisions regarding treatments. Moreover, continuous strengthening of diagnostic capacities within the public health system, along with gradual expansion of private diagnostic services, is presenting easier access to molecular testing, thus enabling the broader adoption of molecular diagnostics across a wide range of clinical applications.
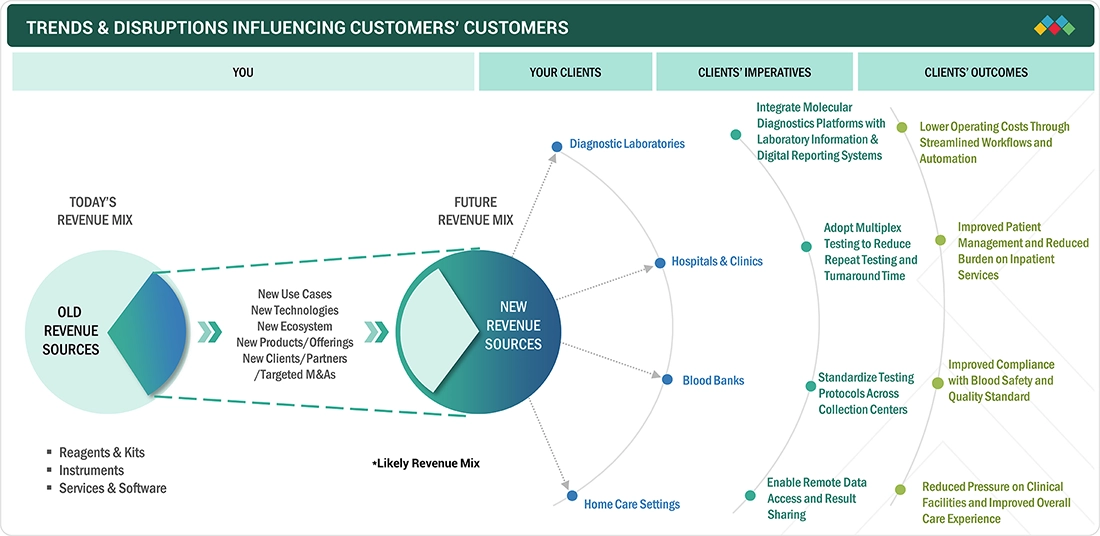
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Latin America molecular diagnostics (MDx) market is evolving as healthcare systems place greater emphasis on improving diagnostic accuracy & efficiency. A gradual shift toward more advanced molecular techniques, including multiplex testing & automated platforms, to support higher testing volumes and standardized results. The increased use of molecular diagnostics in oncology and genetic testing is also shaping market direction. At the same time, disruption is emerging from the growing use of PoC molecular tests and digital tools that simplify workflows, allowing molecular diagnostics to be used beyond traditional centralized settings.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing prevalence of infectious diseases and cancer

-
Surge in technological advancements
Level
-
High cost of molecular diagnostic instruments
-
Inadequate reimbursements
Level
-
Growing significance of companion diagnostics
-
Increasing adoption of automation tools to improve laboratory efficiency
Level
-
Operational barriers and labor shortage
-
Changing regulatory landscape
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Surge in technological advancements
Ongoing technological advancements are becoming increasingly important in promoting the growth of the molecular diagnostics market in Latin America. Improvements in assay sensitivity, automation, and workflow efficiency are making molecular testing more reliable and easier to implement. Newer platforms are designed to handle higher sample volumes while reducing manual intervention, which helps laboratories improve productivity. These technological improvements are gradually encouraging the wider adoption of molecular diagnostics in routine testing environments.
Restraint: High cost of molecular diagnostic instruments
The high cost of molecular diagnostic instruments continues to limit adoption in several parts of the region. Many healthcare facilities face budget constraints, making it difficult to invest in advanced platforms. Beyond initial purchase costs, ongoing expenses related to maintenance, consumables, and upgrades add financial pressure. These cost challenges can slow technology adoption, particularly in smaller laboratories & public healthcare settings.
Opportunity: Growing significance of companion diagnostics
The increasing use of targeted therapies is creating new opportunities for companion diagnostics in the region. Molecular tests that help identify suitable patient populations are becoming more relevant, especially in oncology. As awareness of personalized treatment approaches grows, the demand for companion diagnostic assays is expected to increase. This trend is opening opportunities for test developers to expand molecular testing applications aligned with treatment decisions.
Challenge: Operational barriers and labor shortage
Operational limitations & shortages of skilled personnel remain a challenge for molecular diagnostics adoption. Advanced molecular testing requires trained staff, standardized workflows, and consistent quality control, which are not uniformly available. Limited access to specialized training further affects operational efficiency. Strengthening workforce skills & improving laboratory processes will be important to support reliable and scalable use of molecular diagnostics.
LATIN AMERICA MOLECULAR DIAGNOSTICS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Application of rapid PCR-based molecular platforms for high-throughput infectious disease testing & decentralized diagnostic workflows. | Supports faster test turnaround, improves scalability, and enables efficient deployment across diverse testing environments. |
 |
Integrated molecular diagnostic systems for infectious disease testing, cancer diagnostics, and companion diagnostics in centralized labs. | Delivers high test accuracy, standardized results, and seamless integration across laboratory workflows. |
 |
Use of next-generation sequencing technologies for oncology profiling, genetic analysis, and large-scale genomic testing programs. | Delivers deep genomic insights, supports precision medicine strategies, and enables scalable high-throughput analysis. |
 |
MDx solutions for rapid identification of pathogens & monitoring of healthcare-associated infections (HAIs). | Enables timely detection, supports infection control strategies, and improves disease management outcomes. |
 |
Application of comprehensive molecular diagnostic instruments and assays for infectious disease testing, genetic analysis, and oncology diagnostics. | Supports high test accuracy, scalable testing workflows, and reliable performance across a wide range of applications. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Latin America molecular diagnostics ecosystem consists of key stakeholders and components across the entire value chain, including products & services, test types, sample types, technologies, applications, techniques, clinical applications, and end users. The stakeholders include manufacturers engaged in developing and introducing products, distributors who sell via third parties or e-commerce platforms, and R&D partners offering outsourced development and manufacturing support. End users include hospitals & clinics, diagnostic laboratories, and other end users that apply molecular diagnostics solutions in carrying out diagnostics. These constitute the key stakeholders in the Latin America molecular diagnostics supply chain.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Latin America Molecular Diagnostics Market, By Product & Service
By product & service, the Latin America molecular diagnostics market is segmented into reagents & kits, instruments, and services & software. In 2025, the reagents & kits segment accounted for the largest market share due to their repeated use in every molecular diagnostic test. Unlike instruments, reagents & kits are consumed on a continuous basis, resulting in steady demand. Increasing molecular testing volumes across routine diagnostic applications supports consistent consumption of assay kits and related consumables.
Latin America Molecular Diagnostics Market, By Test Type
By test type, the market is segmented into laboratory tests and PoC tests. In 2025, laboratory tests accounted for the largest market share as molecular diagnostics in Latin America are predominantly conducted through centralized testing facilities. These settings are preferred for handling higher sample volumes, performing complex molecular analyses, and maintaining standardized testing procedures.
Latin America Molecular Diagnostics Market, By Sample Type
Based on sample type, the market includes blood, serum, and plasma; urine; and other sample types. In 2025, the blood, serum, and plasma segment accounted for the largest share, driven by their routine use across a wide range of molecular diagnostic applications. These samples are commonly collected in clinical practice and are suitable for multiple molecular technologies supporting consistent testing demand.
Latin America Molecular Diagnostics Market, By Technology
By technology, the market is segmented into polymerase chain reaction, isothermal nucleic acid amplification technology, DNA sequencing & next-generation sequencing, in situ hybridization, DNA microarrays, and other technologies. In 2025, PCR dominated the market due to its established use, scalability, and suitability for routine molecular testing across the region.
Latin America Molecular Diagnostics Market, By Application
On the basis of application, the market is divided into infectious disease diagnostics, oncology testing, genetic testing, and other applications. In 2025, infectious disease diagnostics accounted for the largest share, supported by ongoing demand for molecular testing to aid in disease identification & monitoring across healthcare systems.
Latin America Molecular Diagnostics Market, By Technique
By technique, the market is segmented into multiplex testing and singleplex testing. In 2025, multiplex testing accounted for the largest market share as it enables the simultaneous detection of multiple targets from a single sample. This approach improves testing efficiency, reduces the overall turnaround time, and optimizes the use of reagents & samples.
Latin America Molecular Diagnostics Market, By Clinical Application
Based on clinical application, the market is segmented into diagnostics and screening. In 2025, the diagnostics segment accounted for the largest share of the market, driven by the widespread use of molecular tests for disease confirmation and clinical decision-making in routine patient care.
Latin America Molecular Diagnostics Market, By End User
By end user, the market is categorized into hospitals & clinics, diagnostic laboratories, and other end users. In 2025, diagnostic laboratories represented the largest share, reflecting their role in handling centralized testing volumes and performing specialized molecular diagnostic procedures.
REGION
Brazil to register highest CAGR in Latin America molecular diagnostics market
Brazil recorded the highest CAGR due to its large patient base, expanding diagnostic infrastructure, and growing adoption of advanced molecular testing. Increased private-sector investment and improving access to modern diagnostics further support faster market growth.

LATIN AMERICA MOLECULAR DIAGNOSTICS MARKET: COMPANY EVALUATION MATRIX
Danaher (Star) is a leading player in the Latin America molecular diagnostics market due to its strong portfolio of well-established molecular testing platforms, supported by continuous innovation & automation. The company benefits from broad assay menus and scalable systems, which drive recurring demand for consumables. Additionally, Danaher’s focus on workflow efficiency and test reliability has enabled it to maintain a strong competitive position across key molecular diagnostics applications.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 0.72 Billion |
| Market Forecast in 2031 (Value) | USD 1.16 Billion |
| Growth Rate | CAGR of 8.8% from 2026–2031 |
| Years Considered | 2024–2031 |
| Base Year | 2024 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Brazil, Mexico, Colombia, Argentina, Peru, Chile, Rest of Latin America |
| Parent & Related Segment Reports |
Molecular Diagnostics Market US Molecular Diagnostics Market Europe Molecular Diagnostics Market APAC Molecular Diagnostics Market Middle East and Africa Molecular Diagnostics Market |
WHAT IS IN IT FOR YOU: LATIN AMERICA MOLECULAR DIAGNOSTICS MARKET REPORT CONTENT GUIDE
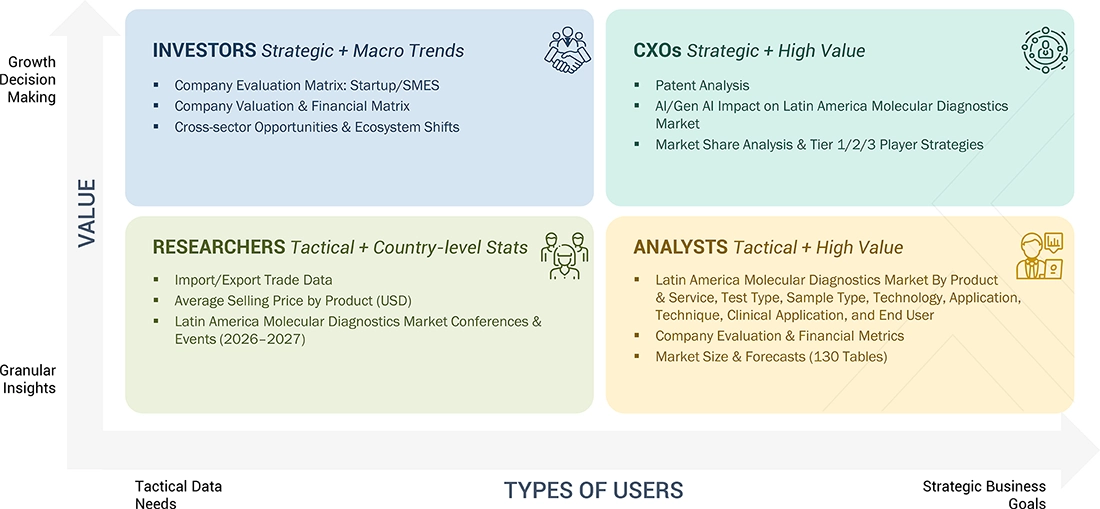
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Product matrix, which provides a detailed comparison of the product portfolio of each company in the Latin America molecular diagnostics market | Enables the easy comparison of competitors’ offerings, helping identify gaps, overlaps, and differentiation opportunities |
| Company Information | Additional five company profiles of players operating in the Latin America molecular diagnostics market | Provides insights into competitors’ strategies, innovation focus, and partnerships, supporting strategic planning |
RECENT DEVELOPMENTS
- February 2025 : bioMérieux (France) received regulatory approval for its BIOFIRE FILMARRAY Gastrointestinal (GI) Panel Mid test. This multiplex molecular test is intended for simultaneous detection of 11 causative bacteria, viruses, and parasites of gastroenteritis in an individual sample, requiring just about an hour for results.
- January 2024 : Roche (Switzerland) launched the cobas Respiratory flex test. The assay is the first diagnostic test to incorporate Roche’s proprietary TAGS (Temperature-activated Generation of Signal) technology, which combines multiplex polymerase chain reaction (PCR) with color, temperature control, and advanced data processing. The test enables detection of up to 15 respiratory pathogens from a single PCR run.
- April 2022 : Illumina, Inc. (US) expanded its commercial operations in Brazil by opening a Solution Center in São Paulo. The center was established to provide customers with access to next-generation sequencing training and technical expertise, supporting improved clinical application and patient outcomes.
Table of Contents

Methodology
The study involved major activities in estimating the current market size for the Latin America Molecular Diagnostics Market. Exhaustive secondary research was done to collect information on the Latin America Molecular Diagnostics Market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, such as top-down and bottom-up, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were used to estimate the market size of the segments and subsegments of the Latin America Molecular Diagnostics Market.
The four steps involved in estimating the market size are as follows:
Secondary Research
In the secondary research process, various secondary sources such as annual reports, press releases & investor presentations of companies, white papers, certified publications, articles by recognized authors, gold-standard & silver-standard websites, regulatory bodies, and databases (such as D&B Hoovers, Bloomberg Business, and Factiva) were referred to, to identify and collect information for this study.
Primary Research
In the primary research process, various sources from the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources mainly include industry experts from the core and related industries and preferred suppliers, manufacturers, distributors, service providers, technology developers, researchers, and organizations related to all segments of this industry’s value chain. In-depth interviews were conducted with primary respondents, including key industry participants, subject-matter experts, C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess prospects.
Market Size Estimation
The top-down and bottom-up approaches were used to estimate and validate the Latin America Molecular Diagnostics Market’s total size. These methods were also used extensively to estimate the size of various subsegments in the market.
- The key players in the industry have been identified through extensive secondary research.
- Primary and secondary research have determined the revenues generated by leading players operating in the Latin America Molecular Diagnostics Market.
- All percentage share, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Data Triangulation
After arriving at the overall market size by applying the process mentioned above, the total market was split into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Molecular diagnostics is a technique used to identify and analyze nucleic acids or proteins at a molecular level. This technique assesses an individual’s genetic makeup to identify a predisposition to any particular disease or condition and diagnose it.
Stakeholders
- Senior Management
- End Users
- Finance/Procurement Department
- R&D Department
Report Objectives
- To define, describe, segment, and forecast the Latin America Molecular Diagnostics Market by product & service, test type, sample type, technology, application, technique, clinical application, end user, and region
- To provide detailed information regarding the major factors (such as drivers, restraints, opportunities, and challenges) influencing market growth
- To analyze the micromarkets1 concerning individual growth trends, prospects, and contributions to the overall Latin America Molecular Diagnostics Market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies2
- To track and analyze company developments, such as product launches & approvals, partnerships, acquisitions, agreements, and other developments
- To benchmark players within the Latin America Molecular Diagnostics Market using the Company Evaluation Matrix framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offerings
- To study the impact of AI/Gen AI on the Latin America Molecular Diagnostics Market, along with the macroeconomic outlook for each region
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Latin America Molecular Diagnostics Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Latin America Molecular Diagnostics Market