The study involved four major activities in estimating the current size of the medical device testing market. Exhaustive secondary research was done to collect information on the market, peer, and parent markets. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
Secondary Research
Various secondary sources have been referred to in the secondary research process for identifying and collecting information important for this study. The secondary sources include annual reports, press releases, and investor presentations of companies; white papers; journals and certified publications; and articles from recognized authors, websites, directories, and databases. Secondary research has been conducted to obtain key information about the industry’s supply chain, the market’s value chain, the total pool of key players, market segmentation according to the industry trends (to the bottom-most level), regional markets, and key developments from market- and technology-oriented perspectives. The secondary data has been collected and analyzed to determine the overall market size, further validated by primary research.
List of major secondary sources
|
Sources
|
Web Link
|
|
International Organization for Standardization (ISO)
|
https://www.iso.org/home.html
|
|
Canadian Standards Association (CSA)
|
https://www.csagroup.org/
|
|
International Safe Transit Association (ISTA)
|
https://ista.org/
|
|
American Society for Testing Materials (ASTM)
|
https://www.astm.org/
|
|
American National Standards Institute (ANSI)
|
https://www.ansi.org/
|
|
Hong Kong Certification Body Accreditation Scheme (HKCAS)
|
https://www.itc.gov.hk/
|
|
Hong Kong Inspection Body Accreditation Scheme (HKIAS)
|
https://www.itc.gov.hk/
|
|
World Bank Group
|
https://www.worldbank.org/
|
Primary Research
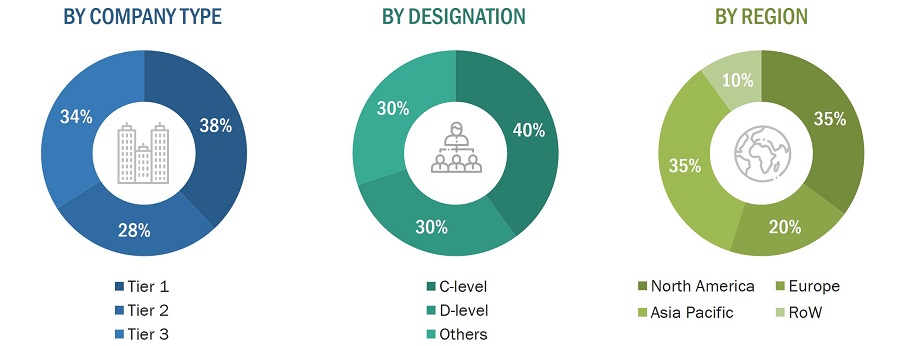
Extensive primary research was conducted after gaining knowledge about the current scenario of the medical device testing market through secondary research. Several primary interviews were conducted with experts from both the demand and supply sides across four major regions—North America, Europe, Asia Pacific, and RoW. This primary data was collected through questionnaires, emails, and telephonic interviews.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
In the complete market engineering process, both top-down and bottom-up approaches have been used, along with several data triangulation methods, to perform market estimation and forecasting for the overall market segments and subsegments listed in this report. Key players in the market have been identified through secondary research, and their market shares in the respective regions have been determined through primary and secondary research. This entire procedure includes the study of annual and financial reports of the top market players and extensive interviews for key insights (quantitative and qualitative) with industry experts (CEOs, VPs, directors, and marketing executives).
All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources. All the parameters affecting the markets covered in this research study have been accounted for, viewed in detail, verified through primary research, and analyzed to obtain the final quantitative and qualitative data. This data has been consolidated and supplemented with detailed inputs and analysis from MarketsandMarkets and presented in this report. The following figure represents this study’s overall market size estimation process.
Bottom-Up Approach
The bottom-up approach was used to arrive at the overall size of the medical device testing market from the revenues of the key players and their shares in the market. The overall market size was calculated based on the revenues of the key players identified in the market.
-
Identifying the entities in the medical device testing market influencing the entire market, along with the related component players
-
Analyzing major providers of medical device testing services, studying their portfolios, and understanding different types of products based on features and functions
-
Analyzing trends pertaining to the usage of medical device testing offerings, such as hardware, software, and services
-
Tracking the ongoing and upcoming developments in the market, such as investments made, R&D activities, product launches, collaborations, and partnerships, and forecasting the market size based on these developments and other critical parameters
-
Carrying out multiple discussions with key opinion leaders to understand the different types of medical device testing; and offerings, vertical, and recent trends in the market, analyzing the breakup of the scope of work carried out by major manufacturing companies
-
Arriving at the market estimates by analyzing segment revenues of these companies generated by medical device testing offerings and then combining the same to get the market estimate by different segments
-
Verifying and cross-checking the estimate at every level by discussing with key opinion leaders, such as Chief Experience Officers (CXOs), directors, and operations managers, and finally with the domain experts at MarketsandMarkets
-
Studying various paid and unpaid sources of information, such as annual reports, press releases, white papers, Factiva, and databases

Top-Down Approach
In the top-down approach, the overall market size has been used to estimate the size of individual markets (mentioned in the market segmentation) through percentage splits from secondary and primary research.
The most appropriate immediate parent market size has been used to implement the top-down approach to calculate the market size of specific segments. The top-down approach has been implemented for the data extracted from the secondary research to validate the market size obtained.
Each company’s market share has been estimated to verify the revenue shares used earlier in the top-down approach. This study has determined and confirmed the overall parent market and individual market sizes by the data triangulation method and data validation through primaries. The data triangulation method in this study is explained in the next section.
-
Focusing on top-line investments and expenditures being made in the ecosystems of various verticals, listing key developments, analyzing updated technology in the marketplace, and evaluating the market by further splitting it into different technology
-
Building and developing the information related to the market revenue generated by key medical device testing tools manufacturers
-
Conducting multiple on-field discussions with the key opinion leaders involved in the development of medical device testing products in various verticals
-
Estimating geographic splits using secondary sources based on various factors, such as the number of players in a specific country and region, the offerings of medical device testing, and the level of solutions offered in different verticals
-
The impact of the recession on the steps mentioned above.

Data Triangulation
After arriving at the overall market size from the estimation process explained above, the overall market has been split into several segments and subsegments. The data triangulation procedure has been employed wherever applicable to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data has been triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the market size has been validated using top-down and bottom-up approaches.
Market Definition
Medical device testing services are provided to help manufacturers improve their productivity, meet the global standards, and enhance the quality of medical devices. Services such as audit, inspection, verification, certification, and testing can be applied to any medical device, service, or company, involved in medical devices. These services aim to assure the end users and consumers that the manufacturer has followed national and international regulations and industry standards to ensure quality, public health, and safety.
Key Stakeholders
-
Raw material and testing equipment suppliers
-
Research organizations
-
Original equipment manufacturers (OEMs)
-
Technology standards organizations, forums, alliances, and associations
-
Technology investors
-
Analysts and strategic business planners
-
Government bodies, venture capitalists, and private equity firms
-
Market Research and Consulting Firms
-
Technology Investors
-
Governments, Regulatory Bodies, and Financial Institutions
-
Venture Capitalists, Private Equity Firms, and Startups
Report Objectives
-
To describe and forecast the medical device testing market, in terms of value, based on service type, sourcing type, device class and, technology.
-
To forecast the market size, in terms of value, for various segments with regard to 4 main regions, namely, North America, Europe, Asia Pacific (APAC), and the Rest of the World (RoW)
-
To provide detailed information regarding the major factors influencing the growth of the medical device testing market (drivers, restraints, opportunities, and industry-specific challenges)
-
To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the total market
-
To study the complete value chain and allied industry segments, and perform a value chain analysis of the medical device testing landscape
-
To analyze the opportunities in the market for various stakeholders by identifying the high-growth segments of the market
-
To profile the key players and comprehensively analyze their market position in terms of ranking and core competencies2, along with detailing the competitive landscape for the market leaders
-
To analyze competitive developments, such as partnerships and joint ventures, mergers and acquisitions, new product developments, expansions, and research and development, in the medical device testing market
-
To track and analyze competitive developments, such as partnerships, collaborations, agreements, joint ventures, mergers and acquisitions, expansions, product/service launches, and other developments in the market
Available Customizations
With the given market data, MarketsandMarkets offers customizations according to the specific requirements of companies. The following customization options are available for the report:
-
Detailed analysis and profiling of additional market players (up to 5)
-
Additional country-level analysis of the medical device testing market
Product Analysis
-
Product matrix, which provides a detailed comparison of the product portfolio of each company in the medical device testing market.

Growth opportunities and latent adjacency in Medical Device Testing Market