
Multi-Lumen Medical Tubing Market
Multi-Lumen Medical Tubing Market by Material (Plastic, Rubber, Specialty Polymers), Application (Bulk Disposable Tubing, Catheters & Cannulas, Drug Delivery Systems), and Country - Global Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
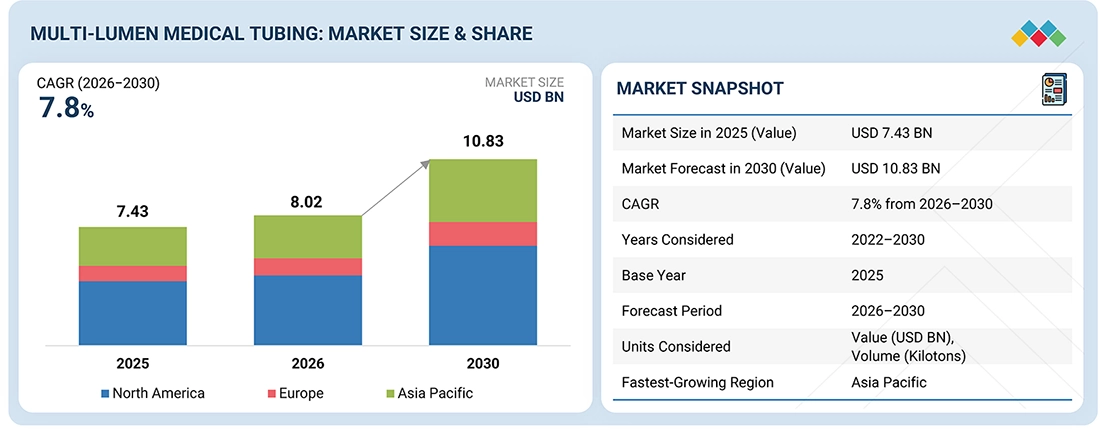
The multi-lumen medical tubing market is projected to grow from USD 8.02 billion in 2026 to USD 10.83 billion by 2030, reflecting a compound annual growth rate (CAGR) of 7.8% during this period. This growth is driven by a rising demand for minimally invasive procedures, an increasing prevalence of chronic diseases, advancements in raw materials and extrusion processes, a shift toward home healthcare, and a growing adoption of disposable medical devices.
KEY TAKEAWAYS
-
By CountryNorth America dominated the multi-lumen medical tubing market, with a share of 40%, in terms of value, in 2025.
-
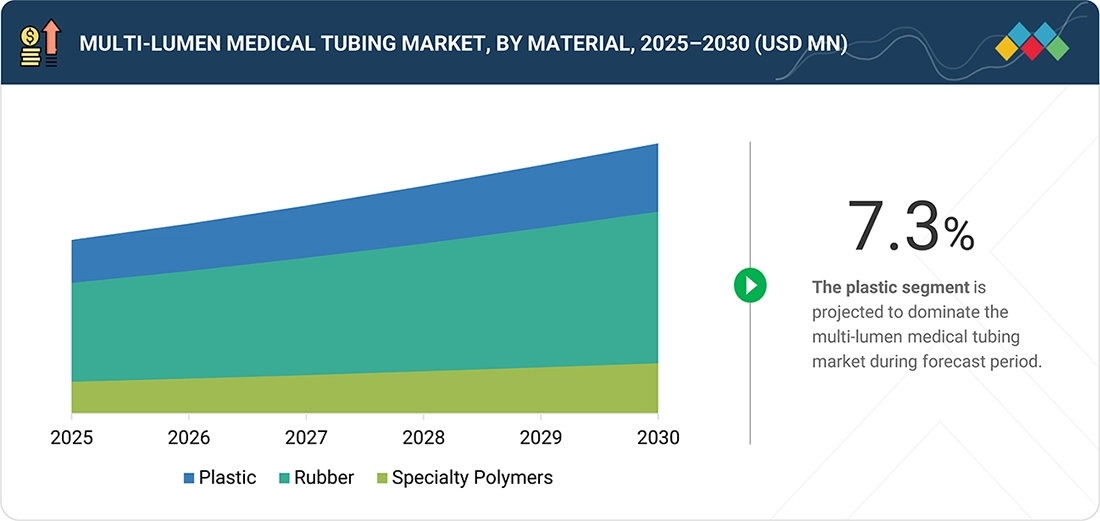
By MaterialBy material, the specialty polymers segment is projected to be the fastest-growing segment during the forecast period. It is projected to grow at a CAGR of 8.6% between 2026 and 2030.
-
By ApplicationBy application, the catheters & cannulas segment is projected to account for the largest market share of 35% in terms of value, during the forecast period.
-
Competitive Landscape - Key StartupsArkema, Lubrizol, Saint-Gobain were identified as some of the star players in the multi-lumen medical tubing market, given their strong market share and product footprint.
-
Competitive Landscape - Startups/SMEs4D Biomaterials, CytexOrtho, and BIOVOX have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The multi-lumen medical tubing market is projected to grow at a significant rate during the forecast period. This growth is driven by an increasing demand for less-invasive devices, the establishment of new hospital facilities, and the rising trend of home healthcare practices, all of which require high-quality and biocompatible tubes. The market is also experiencing significant changes due to new agreements and developments. These include strategic partnerships between original equipment manufacturers (OEMs) and material suppliers, investments in research and development, advancements in technology, and the creation of eco-friendly composites.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The multi-lumen medical tubing market is experiencing consistent growth, primarily driven by an increase in procedure volumes, stricter infection control policies, and a greater reliance on single-use consumables across various healthcare settings, including hospitals, clinics, and home care. Additionally, the establishment of new hospitals in the Asia Pacific region has significantly contributed to the rising demand for high-quality, biocompatible, and cost-effective multi-lumen medical tubing. At the same time, the market is evolving due to partnerships between original equipment manufacturers (OEMs) and suppliers, advancements in extrusion and sterilization technologies, and the introduction of PVC-free materials along with sustainable polymer alternatives.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising demand for minimally invasive procedures

-
Technological advancements in materials and extrusion
Level
-
Environmental concerns
-
Supply chain disruptions and raw material fluctuations
Level
-
Customization and specialized applications
-
Sustainability and regulatory-driven innovations
Level
-
Stringent regulatory requirements
-
Technical manufacturing complexities
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising demand for minimally invasive procedures
The increasing preference for minimally invasive surgeries is driving the growth of the multi-lumen medical tubing sector. Doctors and patients favor these techniques because they involve smaller incisions, lead to faster healing times, and carry fewer risks than traditional surgical methods. As a result, there has been a significant rise in demand for innovative tubing that can perform multiple functions in a compact design. Multi-lumen tubes are designed with separate channels within a single tube structure, allowing for various actions such as fluid delivery, drainage, passing guidewires, or even embedding sensors. This design keeps the overall size smaller while enhancing efficiency during procedures. As the healthcare sector shifts toward safer and quicker options that reduce hospital stays and complications, multi-lumen tubing has emerged as a vital solution. It meets the needs of modern medical interventions while also improving patient outcomes by minimizing device bulk and streamlining procedural steps.
Restraint: Environmental and disposal concerns
Concerns regarding environmental impact and waste disposal are significant obstacles to the growth of the multi-lumen medical tubing market. Hospitals and clinics prefer disposable tubing, including multi-lumen varieties used in catheters and drug delivery systems, to reduce the risk of hospital-acquired infections and maintain sterility. However, this preference results in substantial plastic waste that either remains in landfills or contributes to pollution when incinerated. Traditional materials like PVC do not decompose easily, leading to long-term environmental accumulation and raising concerns about microplastics and their harmful effects on ecosystems. With growing global awareness regarding plastic waste and increasing regulations on single-use plastics—especially in Europe—manufacturers face pressure to adopt sustainable production practices. Developing eco-friendly alternatives, such as bio-based or biodegradable polymers, requires significant investments in research, testing for biocompatibility, and obtaining regulatory approvals, which can increase costs and prolong innovation cycles.
Opportunity: Customization and specialized applications
As healthcare progresses toward personalized medicine, manufacturers can design products tailored to specific needs, including unique anatomical or procedural requirements that vary in lumen shape (such as round, oval, or crescent), size, wall thickness, material, and special features like tapered profiles, braiding for reinforcement, or integrated striping to enhance visibility. This flexibility allows for the creation of complex, multi-functional devices applicable in areas like interventional cardiology, neurology, urology, and drug delivery—where standard tubing is inadequate. Intricate multi-lumen configurations can support a range of functions, such as the passage of a guidewire, injection of contrast, inflation of a balloon, or even integration of sensors within a single, compact tube. These innovations offer multiple advantages, including increased precision, reduced procedural time, and improved patient outcomes. This trend aligns with broader movements in precision medicine, as advancements in co-extrusion and new materials enable developments such as antimicrobial, bioresorbable, and sensor-embedded solutions.
Challenge: Stringent regulatory requirements
Stringent regulatory requirements continue to pose significant challenges for the growth of multi-lumen medical tubing market. For example, the FDA in the US, along with other regulatory bodies, has strict standards regarding the biocompatibility, sterility, safety, and performance of devices that come into direct contact with blood or tissues, such as multi-lumen catheters used in minimally invasive procedures. Additionally, manufacturers must comply with frameworks, such as ISO 10993 for biological evaluation, which requires extensive testing for cytotoxicity, sensitization, irritation, and systemic toxicity. This compliance process typically spans several years and incurs considerable costs in terms of validation, documentation, and clinical data collection.
MULTI-LUMEN MEDICAL TUBING MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Use of new endotracheal tube to avoid incidence of VAP (Ventilator-associated pneumonia) | Improve complexity of mold design and fabrication | Maintain constant pressure within the tube | Provide collection point for oropharyngeal secretions that could easily be drained and cleaned by the hospital’s nursing staff |
 |
Use of multi-lumen medical tubing for hemodynamic monitoring, IV fluids, and medications | Improve workflow with valves | Enable quick addition of devices without multiple insertions | Reduce procedure time and patient risk |
 |
Use of dual-lumen medical catheters with dual channels in single device | Consistent high blood flows for effective dialysis | Low recirculation improving treatment efficiency | Reliable performance in renal units |
 |
Use of double or triple-lumen tubing for infusion, power injection, and blood sampling | High therapy completion rates | Reduce needle usage | Enhance patient comfort | Support home and outpatient care | Improve safety and efficiency |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The multi-lumen medical tubing market ecosystem involves several key participants. It covers raw material suppliers that provide medical-grade polymers, silicones, thermoplastics, and specific additives to high-volume tubing manufacturers. These tubing manufacturers focus on materials that are cost-effective, ready for sterilization, and biocompatible, making them suitable for use in hospitals, home care, diagnostics, and routine clinical procedures. Intermediaries, such as distributors, and end users [Contract Development and Manufacturing Organizations (CDMOs) and medical device Original Equipment Manufacturers (OEMs)] play a significant role in the large-scale assembly, packaging, and transportation of multi-lumen tubing sets to healthcare facilities around the world. Regions like North America, Europe, and Asia Pacific have strong manufacturing bases that not only maintain competitive pricing but also ensure compliance with regulations and facilitate quick shipping. Each participant in this process is essential for sourcing materials to ensure quality mass production and provide disposable tubing for medical and patient care applications.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Multi-Lumen Medical Tubing Market, By Application
By application, the multi-lumen medical tubing market includes bulk disposable tubing, catheters & cannulas, drug delivery systems, and other products. The catheters & cannulas segment is projected to dominate the market, driven by the increasing demand for minimally invasive surgeries. Bulk disposable tubing is preferred for this application due to its high volume usage in routine procedures and its role in infection prevention.
Multi-Lumen Medical Tubing Market, By Material
By material, the multi-lumen medical tubing market covers rubber, plastic, specialty polymers, and other materials. The market for rubber, which includes silicone and thermoplastic elastomers (TPE), is projected to dominate the market during the forecast period. Silicone and thermoplastic elastomers (TPE)share properties, such as flexibility and biocompatibility and are suitable for critical care applications. On the other hand, plastic is a commonly used for disposable item due to its low cost. Additionally, specialty polymers and bioabsorbable materials are employed in precision and drug delivery applications.
REGION
North America is projected to be the second-largest region in the multi-lumen medical tubing market during the forecast period
The multi-lumen medical tubing market in North America is expanding due to the region's advanced healthcare infrastructure, high healthcare spending among the population, the growing demand for minimally invasive procedures, and the significant presence of medical OEMs.
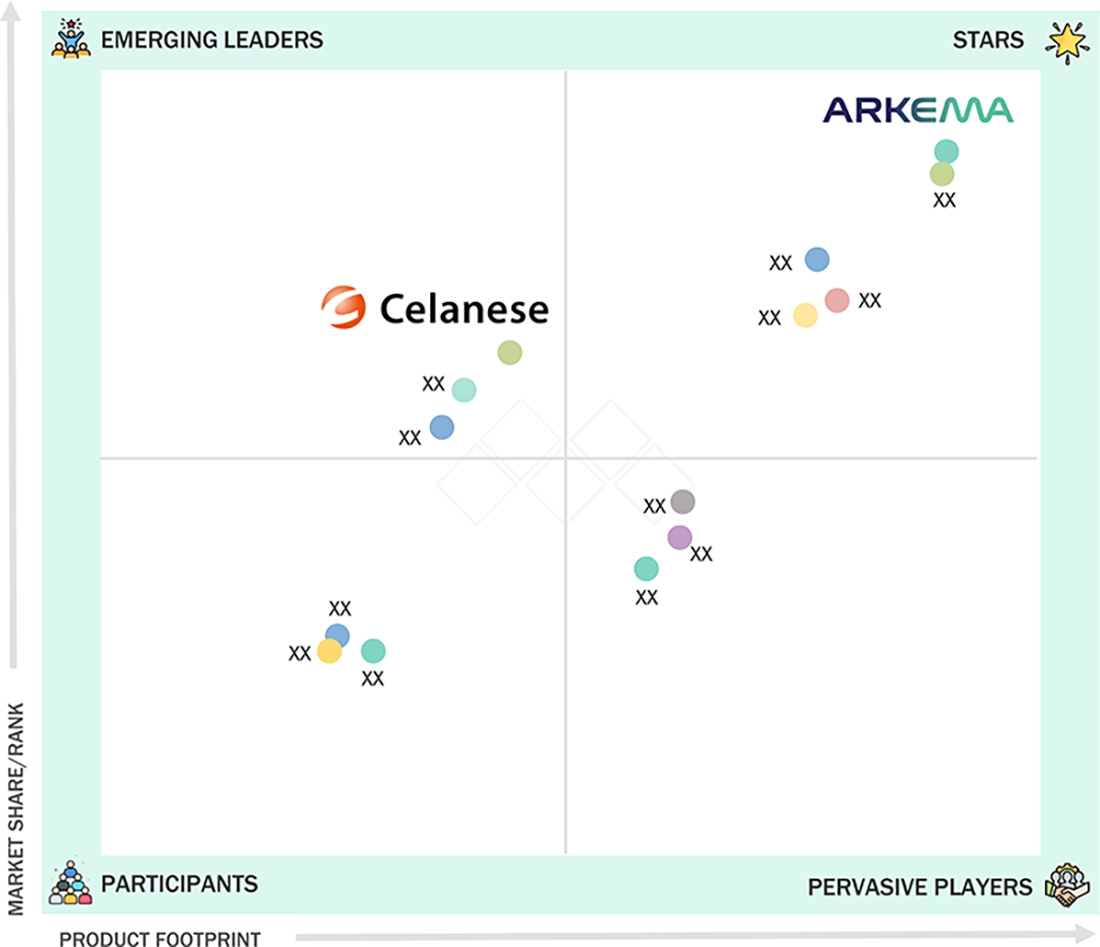
MULTI-LUMEN MEDICAL TUBING MARKET: COMPANY EVALUATION MATRIX
Arkema (Star) has established itself as a leader in the global multi-lumen medical tubing market. Its success can be attributed to its partnerships with major original equipment manufacturers (OEMs) and healthcare providers, along with its outstanding specialty polymers, high-performance resins, and engineered additives tailored for medical tubing. Meanwhile, Celanese Corporation (Emerging Player) is becoming a formidable competitor with its diverse product portfolio and investments in specialty grades for medical applications. The company is also focusing on high-value medical segments, such as controlled-release drug delivery.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- 1. Arkema (France)
- 2. Avient Corporation (US)
- 3. BASF (Germany)
- 4. Celanese Corporation (US)
- 5. Covestro (Germany)
- 6. DuPont (US)
- 7. Evonik (Germany)
- 8. The Lubrizol Corporation (US)
- 9. Saint-Gobain Performance Plastics (France)
- 10. Solvay (Belgium)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 7.43 Billion |
| Market Forecast in 2030 (Value) | USD 10.83 Billion |
| Growth Rate | CAGR of 7.8% from 2026–2030 |
| Years Considered | 2022–2030 |
| Base Year | 2025 |
| Forecast Period | 2026–2030 |
| Units Considered | Value (USD Billion), Volume (Kiloton) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | North America, Asia Pacific, Europe, South America, Middle East & Africa |
WHAT IS IN IT FOR YOU: MULTI-LUMEN MEDICAL TUBING MARKET REPORT CONTENT GUIDE
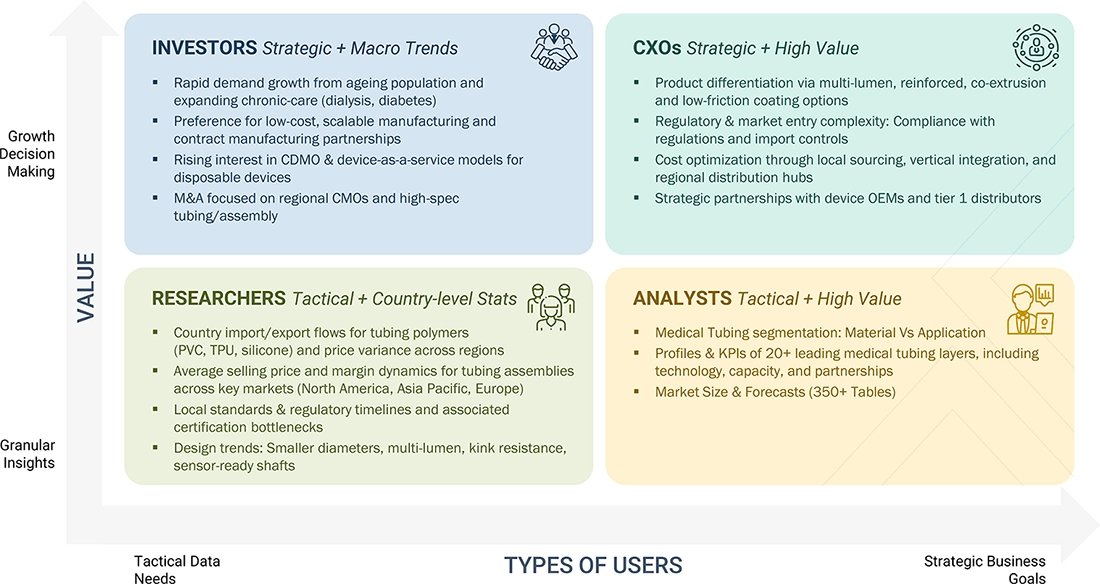
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Global Medical Device OEM |
|
|
| Hospital & Healthcare System Provider (Global) | Pre-sterilized bulk disposable IV/oxygen/suction sets packaged for hospital usage, color-coding and batch-traceability, bulk kitting for inventory management |
|
| Contract Manufacturer/CDMO | Custom tubing reels, cut-to-length assemblies, automated connector bonding, sterile double-bagging, EO/gamma validation support and on-demand production ramps |
|
| Catheter and Cannula Manufacturer | Low-friction inner coatings, tapered tips, reinforced braid options, and coated single-use catheter tubing in standardized reels for downstream assembly |
|
| Drug Delivery & Infusion System Provider | Precision single-lumen tubing for infusion pumps, metered-flow tubing, integrated luer interfaces, and tubing validated for drug compatibility and extractables/leachable |
|
RECENT DEVELOPMENTS
- July 2025 : Arterex (contract medical device manufacturer) acquired Xponent Global, Inc. (extruded tubing manufacturer). Xponent Global provides high-quality precise filaments, over-the-wire extrusion, multi-lumen, taper tubing, para tubing, and tight tolerance single lumen tubing for a wide range of applications. The acquisition aimed at helping Arterex to expand its product offerings.
- November 2024 : Lubrizol signed an MoU with Polyhose to set up a medical-tubing manufacturing facility near Chennai, India. This was a strategic move to multiply local tubing capacity and support Make-in-India supply.
- November 2024 : Teknor Apex introduced new medical-grade TPE compounds (Medalist® series) optimized for biopharma tubing. This development improved clarity, reduced spallation, and pump compatibility for disposable tubing applications.
- May 2024 : Dow announced an MOU with SCGC to convert 200 ktpa of plastic waste into circular products, supporting development of PVC-free and recycled polymer solutions applicable to high-volume medical tubing.
- February 2022 : Zeus added PTFE Sub-Lite-Wall™ multi-lumen tubing to its product portfolio. The new product features high structural integrity, improved planarity, high lubricity, and excellent dielectric strength. Zeus can produce PTFE Sub-Lite-Wall multi-lumens with average max wall thicknesses ranging from 0.002” to 0.005” (0.051 mm to 0.127 mm).
Table of Contents

Methodology
The study involved four major activities for estimating the current size of the europe medical tubing market. Exhaustive secondary research was conducted to collect information on the market, the peer product market, and the parent product group market. The next step was to validate these findings, assumptions, and sizes with the industry experts across the value chain of europe medical tubing through primary research. Both the top-down and bottom-up approaches were employed to estimate the overall size of the europe medical tubing market. After that, market breakdown and data triangulation procedures were used to determine the size of different segments and sub-segments of the market.
Secondary Research
In the secondary research process, various secondary sources such as Hoovers, Factiva, Bloomberg BusinessWeek, and Dun & Bradstreet were referred, to identify and collect information for this study on the europe medical tubing market. These secondary sources included annual reports, press releases & investor presentations of companies; white papers; certified publications; articles by recognized authors; regulatory bodies, trade directories, and databases.
Primary Research
The europe medical tubing market comprises several stakeholders in the supply chain, which include raw material suppliers, distributors, end-product manufacturers, buyers, and regulatory organizations. Various primary sources from the supply and demand sides of the markets have been interviewed to obtain qualitative and quantitative information. The primary participants from the demand side include key opinion leaders, executives, vice presidents, and CEOs of companies in the europe medical tubing market. Primary sources from the supply side include associations and institutions involved in the europe medical tubing market, key opinion leaders, and processing players.
Market Size Estimation
The bottom-up and top-down approaches have been used to estimate the europe medical tubing market by material, application, structure, and region. The research methodology used to calculate the market size includes the following steps:
- The key players in the industry and markets were identified through extensive secondary research.
- In terms of value, the industry’s supply chain and market size were determined through primary and secondary research processes.
- All percentage shares, splits, and breakdowns were determined using secondary sources and verified through primary sources.
- All possible parameters that affect the markets covered in this research study were accounted for, viewed in extensive detail, verified through primary research, and analyzed to obtain the final quantitative and qualitative data.
- The research included studying reports, reviews, and newsletters of top market players and extensive interviews with leaders such as directors and marketing executives to obtain opinions.
The following figure illustrates the overall market size estimation process employed for this study.
Data Triangulation
After arriving at the overall size of the europe medical tubing market from the estimation process explained above, the total market was split into several segments and sub-segments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Along with this, the market size was validated using both the top-down and bottom-up approaches.
Market Definition
Europe medical tubing is flexible, hollow tubing used in a range of medical and pharmaceutical uses for the transfer of fluids and gases. Europe medical tubing is a critical component in healthcare environments for uses including drug delivery, respiratory therapy, catheters, intravenous (IV) therapy, and peristaltic pumps. Europe medical tubing is produced from materials such as silicone, polyvinyl chloride (PVC), thermoplastic elastomers (TPE), and polyethylene, providing biocompatibility, chemical resistance, and flexibility. The market for europe medical tubing is growing due to improving healthcare spending, expanding demand for minimally invasive treatments, and technological innovation in medical devices. The growing prevalence of chronic diseases, such as cardiovascular diseases and diabetes, has also fueled demand for europe medical tubing solutions.
Stakeholders
- Europe Medical Tubing Manufacturers
- Raw Material Suppliers
- Regulatory Bodies and Government Agencies
- Distributors and Suppliers
- End-Use Industries
- Associations and Industrial Bodies
- Market Research and Consulting Firms
Report Objectives
- To define, describe, and forecast the size of the europe medical tubing market in terms of value and volume.
- To provide detailed information regarding the key factors influencing the growth of the market (drivers, restraints, opportunities, and challenges).
- To forecast the market size based on material, application, structure, and region.
- To forecast the market size for the five main regions—North America, Europe, Asia Pacific (APAC), South America, and the Middle East & Africa (MEA),—along with their key countries.
- To strategically analyze micro markets with respect to individual growth trends, prospects, and contributions to the total market.
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for the market leaders.
- To strategically profile leading players and comprehensively analyze their key developments such as new product launches, expansions, and deals in the europe medical tubing market.
- To strategically profile key players and comprehensively analyze their market shares and core competencies.
- To study the impact of AI/Gen AI on the market under study, along with the macroeconomic outlook.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Multi-Lumen Medical Tubing Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Multi-Lumen Medical Tubing Market