
North America Medical Tubing Market
North America Medical Tubing Market by Application (Bulk Disposable Tubing, Catheter & Cannula, Drug Delivery System), By Material (Plastic, Rubber, Specialty Polymer), Structure (Single-Lumen, Co-Extruded, Multi-Lumen, Tapered/Bump Tubing, Braided Tubing), Region - Global Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
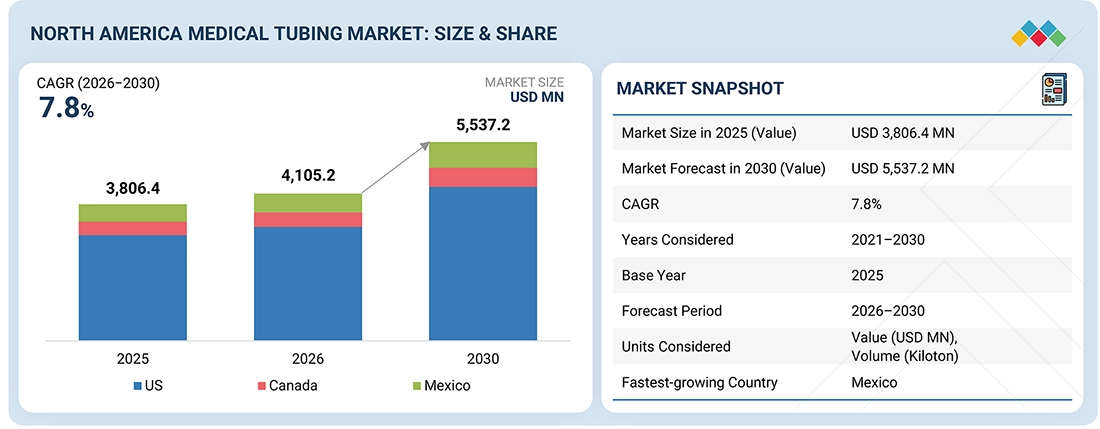
The North America medical tubing market is projected to reach USD 5,537.2 million by 2030 from USD 4,105.2 million in 2026, at a CAGR of 7.8% from 2026 to 2030. The North America medical tubing market is expanding steadily due to increasing demand for high-performance medical devices, a rising burden of chronic and age-related diseases, and the rapid shift toward home-based and minimally invasive treatments. Growth is further fueled by innovation in biocompatible and high-durability materials, a growing elderly population, strong healthcare infrastructure, and stringent regulatory requirements that encourage continuous improvements in product quality and safety.
KEY TAKEAWAYS
-
By CountryThe US accounts for 77.2% share of the North America medical tubing market in 2025.
-
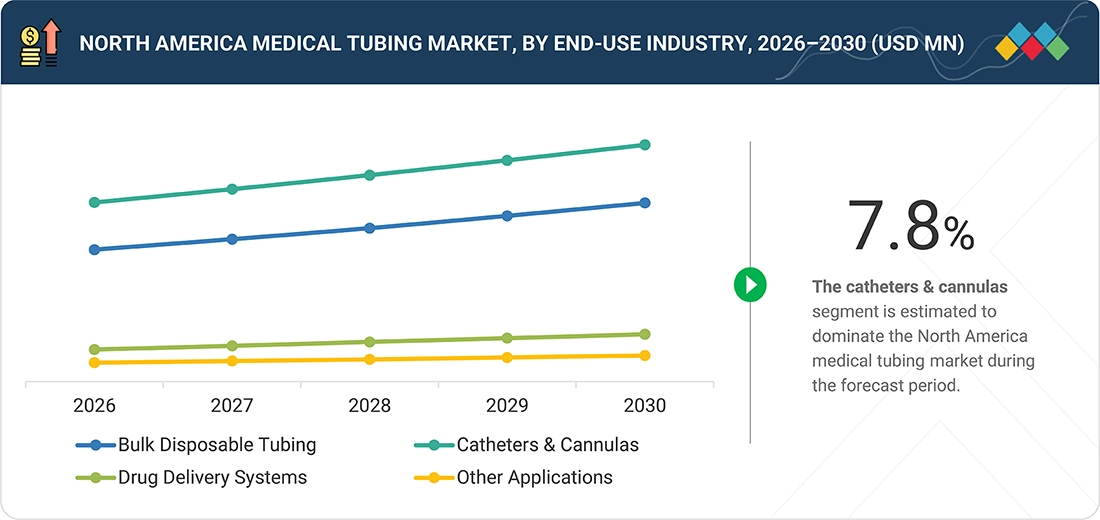
By ApplicationBy application type, the drug delivery systems is expected to register the highest CAGR of 10.3% during the forecast period.
-
By Material TypeBy material type, the speciality polymers segment is expected to register a CAGR of 9.3% during the forecast period.
-
By StructureBy structure, the multi-lumen segment will dominate the market during the forecast period.
-
Competitive Landscape - Key PlayersFreudenberg Medical, Lubrizol and Gore were identified as some of the star players in the medical tubing market (North America), given their strong market share and product footprint.
-
Competitive Landscape - StartupsTeel Plastics, Vesta Inc, Newage Industries among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The North America medical tubing market is experiencing strong growth driven by rising demand for advanced medical devices, increasing incidence of chronic and age-related conditions, and the rapid expansion of home healthcare and minimally invasive treatments. In the United States, high healthcare spending, early adoption of innovative medical technologies, and strong presence of leading medical device manufacturers are key growth drivers. In Canada, government-supported healthcare infrastructure upgrades and growing investments in hospital and diagnostic facilities are boosting demand, while Mexico benefits from expanding medical manufacturing hubs and increasing exports of medical devices to the U.S., supporting regional market expansion
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The North America medical tubing market is reshaped by rapid technological advancements and evolving healthcare delivery models, creating significant trends and disruptions across the industry. Innovations such as multi-lumen and microbore tubing, antimicrobial and drug-eluting coatings, and the use of advanced biocompatible polymers, including TPU, TPE, and silicone, are enhancing safety, performance, and patient comfort. At the same time, stricter FDA and regulatory compliance requirements, growing demand for home healthcare devices, and the shift toward portable and wearable medical technologies are disrupting traditional manufacturing and supply chains, pushing companies to adopt automation, digital quality control, and sustainable, DEHP-free materials to remain competitive in the North American market
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Growing geriatric population in North America

-
Advancements in extrusion technology
Level
-
Environmental disposable concerns
Level
-
Increasing US government healthcare expenditure
-
Advancements in biocompatible and smart materials
Level
-
High production cost and marketing complexities
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Growing geriatric population in North America
The medical tubing market is experiencing significant growth due to the continuously increasing geriatric population in North America. With advancing age, the population becomes highly vulnerable to various chronic, degenerative, and lifestyle-related diseases that need continuous medical intervention and monitoring. Thus, older adults are more frequently subjected to hospital admissions, surgeries, and other long-term therapies, which increases the application of infusion lines, urinary catheters, dialysis tubing, enteral feeding tubes, and respiratory support systems. Additionally, the rapid growth in home healthcare services within the US and Canada is driving a higher demand for portable, lightweight, and easy-to-manage medical tubing systems that support home dialysis, oxygen therapy, and remote patient monitoring. Governments and healthcare providers are investing heavily in geriatric care infrastructure, assisted living facilities, and long-term care centers, thereby driving sustained demand for high-performance, biocompatible medical tubing solutions across North America.
Restraint: Environmental disposable concerns
Environmental and disposable concerns are a key restraint in the North America medical tubing market due to the heavy reliance on single-use, plastic-based products that generate large volumes of medical waste. Growing awareness of plastic pollution, landfill pressure, and carbon footprint is leading to stricter environmental regulations and sustainability targets in hospitals, which are increasing pressure on manufacturers to develop recyclable, biodegradable, or low-impact materials. Additionally, the safe disposal of contaminated medical tubing is costly and highly regulated, increasing operational and compliance burdens for healthcare providers and manufacturers, which can slow product adoption and raise overall market costs
Opportunity: Increasing US government healthcare expenditure
Rising US government healthcare expenditure presents a strong growth opportunity for the North America medical tubing market as higher federal and state funding supports the expansion of hospital infrastructure, outpatient facilities, and public health programs. Increased investments through Medicare, Medicaid, and public health initiatives are driving greater adoption of advanced medical devices, which rely heavily on high-quality medical tubing. Moreover, opportunities arise from government-backed funding for medical technology innovation, infection control, and patient safety programs, leading to higher demand for antimicrobial-coated, biocompatible, and precision-engineered tubing. Public investments in rural healthcare access, home healthcare services, emergency preparedness, and pandemic response infrastructure are further boosting the use of disposable and specialty tubing systems, creating long-term growth potential for manufacturers and suppliers.
Challenge: High production cost and marketing complexities
High production costs and operational complexities represent a major challenge for the North America medical tubing market. Manufacturing medical-grade tubing requires the use of premium biocompatible raw materials, precision extrusion technologies, and strict cleanroom environments, all of which significantly increase capital and operating expenses. Compliance with stringent regulatory standards, such as those of the FDA, ISO, and USP Class VI, also adds cost through extensive testing, documentation, and validation processes. In addition, the need for customized tubing designs, multi-lumen structures, tight dimensional tolerances, and advanced surface coatings increases manufacturing complexity, leading to longer production cycles and a higher risk of defects. Skilled labor requirements, equipment maintenance, and supply chain disruptions further challenge manufacturers, limiting scalability and increasing overall operational burden
NORTH AMERICA MEDICAL TUBING MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Manufactures precision-engineered medical tubing for fluid transfer, minimally invasive procedures, and critical care applications in regulated North American healthcare environments | High biocompatibility | Tight dimensional tolerances | Reduced risk of leakage | Improved patient safety |
 |
Designs high-performance, PTFE-based medical tubing for complex and critical clinical applications requiring exceptional purity and mechanical strength | High purity materials | Excellent kink resistance Long service life | Enhanced patient comfort |
 |
Develops advanced polymer-based tubing solutions using high-performance materials such as TPU and specialty resins for next-generation medical devices | Superior flexibility | Chemical resistance | Enhanced durability | Compatibility with regulatory standards |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The North America medical tubing market ecosystem consists of raw material suppliers (Dupont, Robinson), manufacturers (Freudenberg Medical, Lubrizol), and end users (Mayo Clinic, Cleveland Clinic). The value chain begins with sourcing medical-grade polymers, including PVC, silicone, polyurethane, polyethylene, and specialty elastomers. These materials are processed through precision extrusion, co-extrusion, and molding technologies to produce single-lumen and multi-lumen tubing with strict dimensional control. Product development involves rigorous testing for biocompatibility, sterility, chemical resistance, flexibility, and pressure tolerance. Distributors and OEM partners then supply these tubing products to hospitals, diagnostic centers, medical device manufacturers, and home healthcare providers, where they are integrated into IV sets, catheters, dialysis systems, respiratory devices, and drug delivery systems.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Medical Tubing Market, By Application
Catheters and cannulas represent the largest application segment in the North America medical tubing market due to their critical role in drug delivery, fluid management, diagnostics, and minimally invasive procedures. These products rely heavily on high-quality medical tubing that offers flexibility, kink resistance, biocompatibility, and precise flow control, making them essential for procedures such as urinary catheterization, cardiovascular interventions, IV access, and surgical drainage. The high prevalence of chronic diseases, an aging population, and the increasing number of surgical and interventional procedures in the US and Canada are driving sustained demand for advanced catheter and cannula systems. Additionally, growing adoption of minimally invasive surgeries, expansion of outpatient and ambulatory care centers, and continuous innovations in coated and antimicrobial tubing technologies are further strengthening the dominance of this application segment in the North America market.
Medical Tubing Market, By Material
Rubber holds the largest share, by material, in the North America medical tubing market due to its excellent flexibility, elasticity, and biocompatibility, making it ideal for critical medical applications. Silicone rubber and natural/synthetic rubber-based compounds are widely used in catheters, cannulas, infusion tubing, respiratory circuits, and peristaltic pump systems as they provide superior kink resistance, soft touch, and patient comfort. The dominance of rubber materials is further supported by their high thermal stability, chemical resistance, and sterilization compatibility (autoclave, gamma, and ethylene oxide). The growing demand for high-performance, long-duration implantable and non-implantable devices, along with increased focus on patient safety and comfort in North America, continues to drive strong adoption of rubber-based medical tubing.
Medical Tubing Market, By Structure
Multilumen tubing holds the largest share, by structure, of the North America medical tubing market due to its ability to simultaneously deliver multiple fluids, drugs, or gases through a single compact tube. This design is critical in advanced medical applications, such as catheters, minimally invasive surgical devices, cardiovascular interventions, and critical care systems, where precise flow control and space efficiency are essential. The growing demand for minimally invasive procedures, complex drug delivery systems, and critical care monitoring in the US and Canada is significantly driving the adoption of multilumen tubing. Its advantages, including improved functionality, reduced infection risk by minimizing connections, enhanced patient comfort, and streamlined device design, make it the preferred structure type in the North America market.
REGION
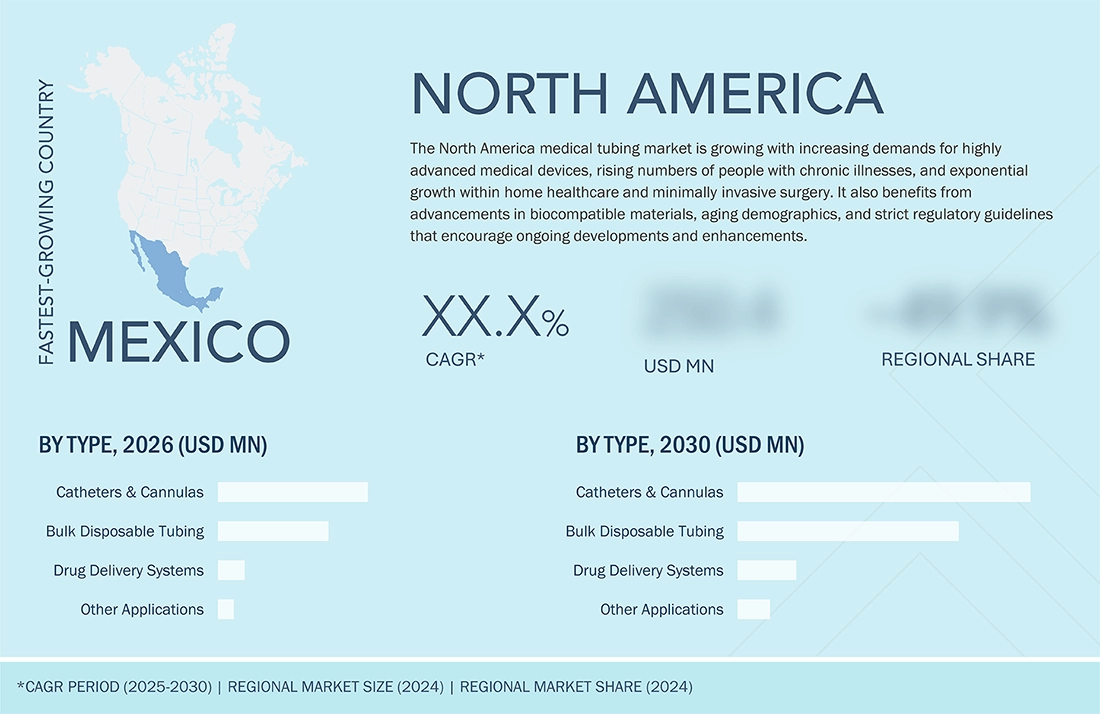
Mexico to be fastest-growing country in North America medical tubing market during forecast period
Mexico is the fastest-growing country in the North America medical tubing market due to its rapidly expanding medical device manufacturing industry, increasing foreign direct investment (FDI), and strong integration with the US healthcare supply chain. The country offers lower production costs, a skilled manufacturing workforce, and favorable trade agreements, which attract global medical device companies to establish production facilities. In addition, rising healthcare infrastructure development, growing hospital investments, and increasing demand for disposable and minimally invasive medical devices are accelerating the adoption of advanced medical tubing. Government support for the medical technology sector and the expansion of export-oriented manufacturing hubs further position Mexico as a high-growth market in the region.
NORTH AMERICA MEDICAL TUBING MARKET: COMPANY EVALUATION MATRIX
In the North America medical tubing market matrix, Lubrizol (Star) leads with a strong market share and a broad medical-grade tubing product portfolio, driven by its advanced polymer technologies, biocompatible materials, and high-performance solutions used in catheters, IV lines, and minimally invasive medical devices. Teknor Apex (Emerging Leader) is rapidly gaining traction in the medical tubing market due to its focus on innovative thermoplastic elastomer compounds, enhanced flexibility and durability, and its expanding manufacturing and distribution footprint across North America.
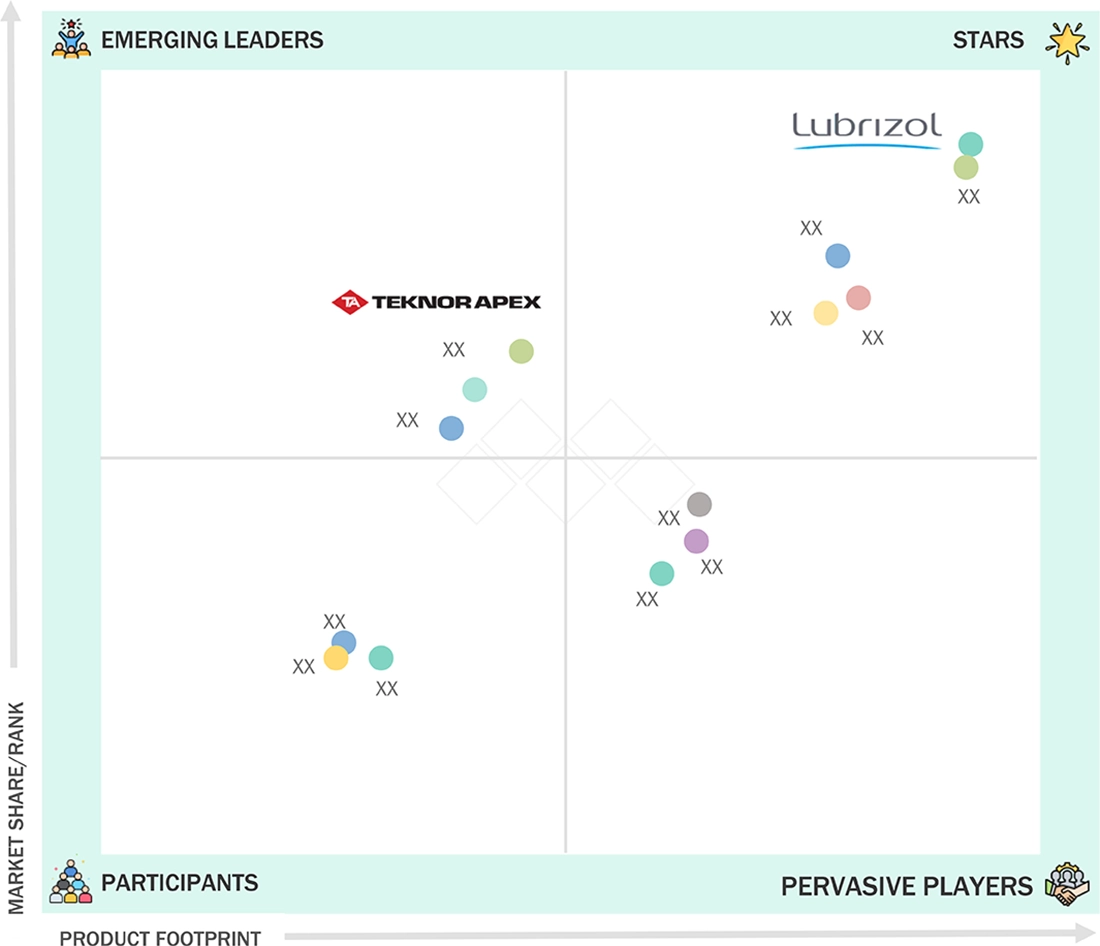
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Freudenberg Medical (US)
- Lubrizol Corporation (US)
- W.L. Gore & Associates Inc. (US)
- Nordson Corporation(US)
- Teknor Apex (US)
- Spectrum Plastics Group (US)
- Zeus Company LLC (US)
- FBK Medical Tubing (US)
- Proteal Cable America Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 3,806.4 Million |
| Market Forecast in 2030 (Value) | USD 5,537.2 Million |
| Growth Rate | CAGR of 7.8% from 2026-2030 |
| Years Considered | 2021-2030 |
| Base Year | 2022 |
| Forecast Period | 2026-2030 |
| Units Considered | Value (USD Million/Billion), Volume (Kiloton) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | US, Canada, Mexico |
WHAT IS IN IT FOR YOU: NORTH AMERICA MEDICAL TUBING MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Demand for country-specific market insights | Provided a detailed breakdown of medical tubing demand, pricing trends, regulatory landscape, and key end-use sectors for target countries |
|
| Request for competitor benchmarking | Delivered a comparison of top North American medical tubing manufacturers, focusing on product portfolios, capacity, pricing, R&D focus, and strategic initiatives |
|
RECENT DEVELOPMENTS
- August 2024 : Nordson Corporation acquired Atrion Corporation, a leader in medical infusion and cardiovascular solutions, to expand its medical portfolio into new markets. This strategic move aligns with long-term growth trends and enhances Nordson’s precision technology capabilities. Atrion’s expertise complements Nordson’s offerings and strengthens its position in the medical industry.
- Feb-24 : Freudenberg Medical expanded its manufacturing site by establishing a second production facility in Costa Rica. The company invested USD 25 million to meet the growing global demand for precision components and medical devices.
- Feb-23 : Lubrizol Corporation's Life Science Health expanded its medical device design center in Corona, California. This expansion helped the company to enhance customer collaboration in every phase of product development. This center offers high-quality medical devices such as precision extrusion, braid and coil-reinforced catheter designs, balloon design, bonding, folding & pleating, and delivery systems.
- September 2020 : Freudenberg Medical, LeLC acquired the dedicated manufacturing equipment and associated assets utilized by Merit Medical Systems, Inc. in its hypotube manufacturing business. This development helped the company nhancein enhancing its production capacity of hypotubes.
- June 2020 : Nordson Corporation acquired Fluortek, Inc., a precision plastic extrusion manufacturer that specializes in medical tubing components for complex medical devices. The acquisition of Fluortek, Inc. expanded Nordson Corporation's medical tubing offerings for complex medical devices.
Table of Contents

Methodology
The study involved four major activities for estimating the current size of the europe medical tubing market. Exhaustive secondary research was conducted to collect information on the market, the peer product market, and the parent product group market. The next step was to validate these findings, assumptions, and sizes with the industry experts across the value chain of europe medical tubing through primary research. Both the top-down and bottom-up approaches were employed to estimate the overall size of the europe medical tubing market. After that, market breakdown and data triangulation procedures were used to determine the size of different segments and sub-segments of the market.
Secondary Research
In the secondary research process, various secondary sources such as Hoovers, Factiva, Bloomberg BusinessWeek, and Dun & Bradstreet were referred, to identify and collect information for this study on the europe medical tubing market. These secondary sources included annual reports, press releases & investor presentations of companies; white papers; certified publications; articles by recognized authors; regulatory bodies, trade directories, and databases.
Primary Research
The europe medical tubing market comprises several stakeholders in the supply chain, which include raw material suppliers, distributors, end-product manufacturers, buyers, and regulatory organizations. Various primary sources from the supply and demand sides of the markets have been interviewed to obtain qualitative and quantitative information. The primary participants from the demand side include key opinion leaders, executives, vice presidents, and CEOs of companies in the europe medical tubing market. Primary sources from the supply side include associations and institutions involved in the europe medical tubing market, key opinion leaders, and processing players.
Market Size Estimation
The bottom-up and top-down approaches have been used to estimate the europe medical tubing market by material, application, structure, and region. The research methodology used to calculate the market size includes the following steps:
- The key players in the industry and markets were identified through extensive secondary research.
- In terms of value, the industry’s supply chain and market size were determined through primary and secondary research processes.
- All percentage shares, splits, and breakdowns were determined using secondary sources and verified through primary sources.
- All possible parameters that affect the markets covered in this research study were accounted for, viewed in extensive detail, verified through primary research, and analyzed to obtain the final quantitative and qualitative data.
- The research included studying reports, reviews, and newsletters of top market players and extensive interviews with leaders such as directors and marketing executives to obtain opinions.
Data Triangulation
After arriving at the overall size of the europe medical tubing market from the estimation process explained above, the total market was split into several segments and sub-segments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Along with this, the market size was validated using both the top-down and bottom-up approaches.
Market Definition
Europe medical tubing is flexible, hollow tubing used in a range of medical and pharmaceutical uses for the transfer of fluids and gases. Europe medical tubing is a critical component in healthcare environments for uses including drug delivery, respiratory therapy, catheters, intravenous (IV) therapy, and peristaltic pumps. Europe medical tubing is produced from materials such as silicone, polyvinyl chloride (PVC), thermoplastic elastomers (TPE), and polyethylene, providing biocompatibility, chemical resistance, and flexibility. The market for europe medical tubing is growing due to improving healthcare spending, expanding demand for minimally invasive treatments, and technological innovation in medical devices. The growing prevalence of chronic diseases, such as cardiovascular diseases and diabetes, has also fueled demand for europe medical tubing solutions.
Stakeholders
- Europe Medical Tubing Manufacturers
- Raw Material Suppliers
- Regulatory Bodies and Government Agencies
- Distributors and Suppliers
- End-Use Industries
- Associations and Industrial Bodies
- Market Research and Consulting Firms
Report Objectives
- To define, describe, and forecast the size of the europe medical tubing market in terms of value and volume.
- To provide detailed information regarding the key factors influencing the growth of the market (drivers, restraints, opportunities, and challenges).
- To forecast the market size based on material, application, structure, and region.
- To forecast the market size for the five main regions—North America, Europe, Asia Pacific (APAC), South America, and the Middle East & Africa (MEA),—along with their key countries.
- To strategically analyze micro markets with respect to individual growth trends, prospects, and contributions to the total market.
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for the market leaders.
- To strategically profile leading players and comprehensively analyze their key developments such as new product launches, expansions, and deals in the europe medical tubing market.
- To strategically profile key players and comprehensively analyze their market shares and core competencies.
- To study the impact of AI/Gen AI on the market under study, along with the macroeconomic outlook.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the North America Medical Tubing Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation












Growth opportunities and latent adjacency in North America Medical Tubing Market