
Ophthalmology Drugs Market Size, Growth, Share & Trends Analysis
Ophthalmology Drugs Market by Molecule (Faricimab, Aflibercept, Ranibizumab), Modality (Monoclonal Antibodies & Fusion Proteins), RoA (Intravitreal, Topical), Indication (AMD, Diabetic Retinopathy (DR), DME), End User - Global Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
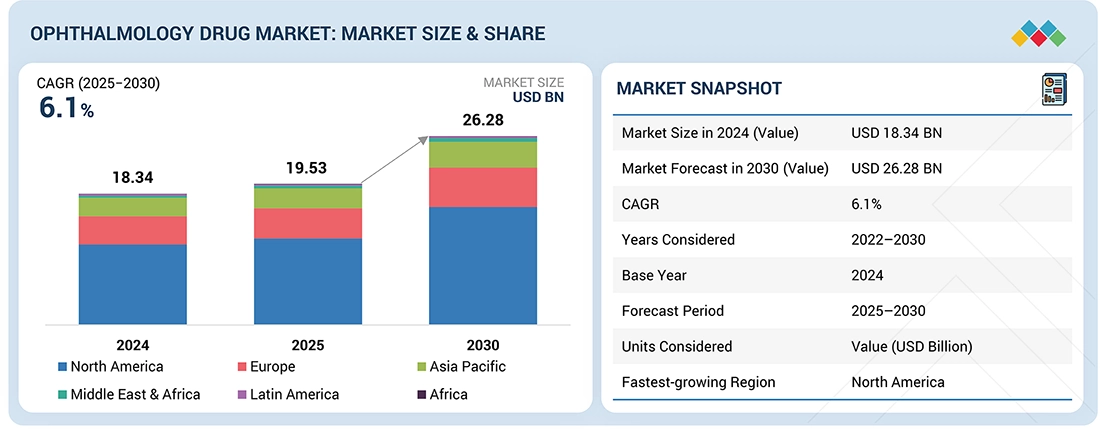
The ophthalmology drug market is projected to reach USD 26.28 billion by 2030 from USD 19.52 billion in 2025, at a CAGR of 6.1% from 2025 to 2030. The growth of the ophthalmology drug market is driven by the increasing population affected by eye conditions, the efficiency of existing drugs, and a strong pipeline of new drugs being developed for ophthalmology indications.
KEY TAKEAWAYS
- The North America ophthalmology drugs market accounted for a 61.1% revenue share in 2024.
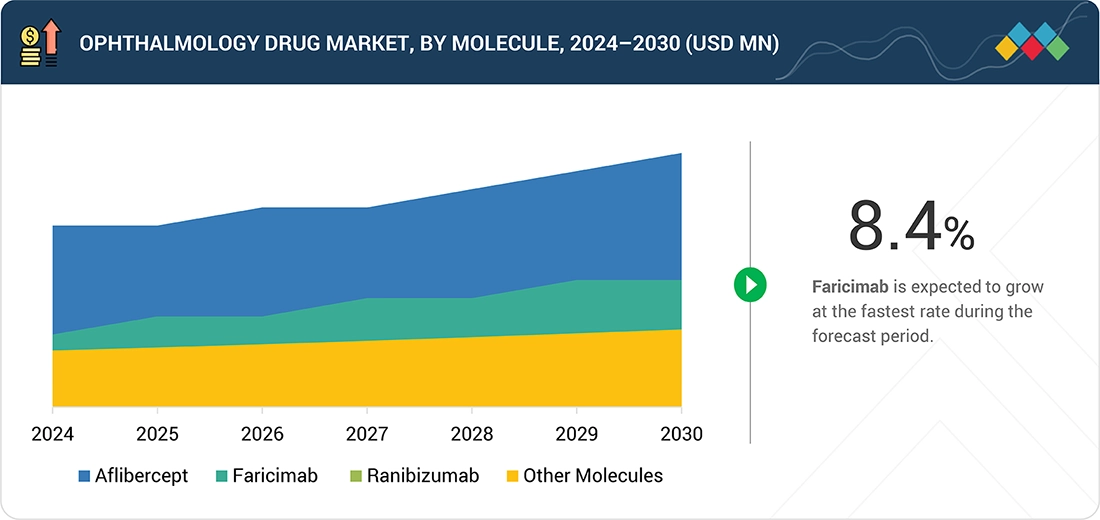
- By molecule, the faricimab segment is expected to register the highest CAGR of 8.4%
- By modality, the mAbs and fusion protein segment accounted for a 84.3% revenue share in 2024, in the ophthalmology drugs market.
- By indication, the Age Related Macular Degeneration (AMD) segment is expected to dominate the market.
- By route of administration, the intravitreal segment is expected to dominate the ophthalmology drugs market.
- By end user, the hospital segment will grow the fastest during the forecast period.
- Company Regeneron Pharmaceuticals Inc., F. Hoffman La- Roche Ltd., and Bayer AG were identified as some of the star players in the Ophthalmology drugs market (global), given their strong market share and product footprint.
- Companies RemeGen, Ocular Therapeutix, Inc. and Adverum Biotechnologies, Inc. among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The ophthalmology drug market is witnessing steady growth, driven by rising investments and strategic collaborations for innovation, advancements in therapy development against ophthalmological indications, and a strong pipeline of drugs being developed for ophthalmological indications. New deals and developments, including strategic partnerships and investments in innovative technologies, are reshaping the industry landscape.
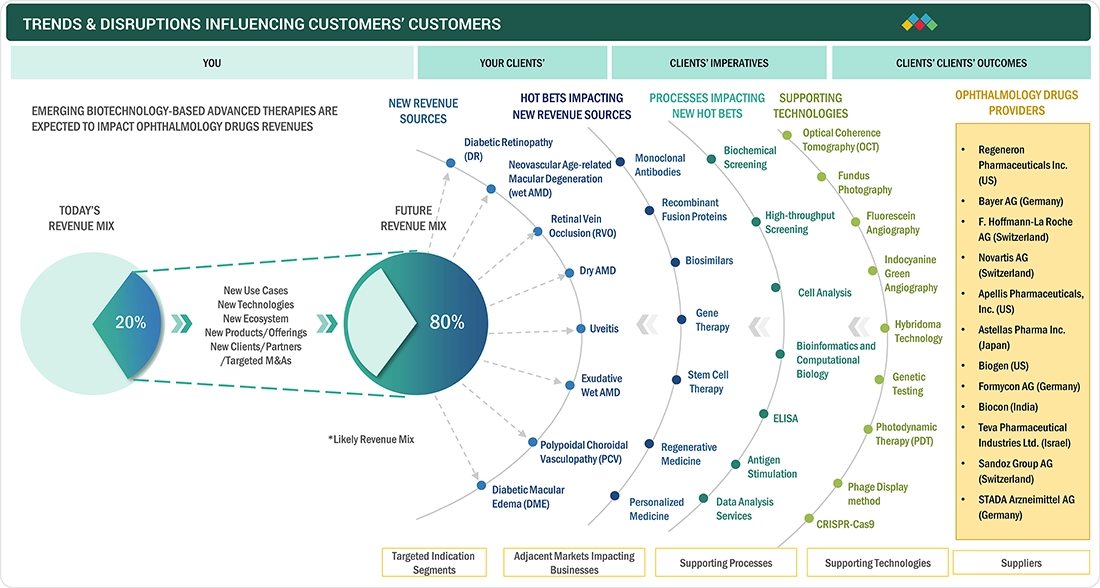
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The impact on patients and healthcare providers emerges from evolving treatment trends and medical disruptions. Hotbets are key pharmaceutical companies in the ophthalmology drugs market, targeting applications that include healthcare providers, hospitals, and specialty clinics that prescribe or administer these therapies. Shifts, such as advances in biologics, gene therapies, or sustained-release drug delivery, will impact treatment outcomes and costs for end users (patients and healthcare systems). The revenue impact on these end users will, in turn, affect the revenues of healthcare providers, which will ultimately influence the revenues of pharmaceutical companies in the ophthalmology drugs market.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Aging population and growing prevalence of vision disorders

-
Strong drug pipeline and growing innovation efforts
Level
-
Off-label drug use for various indications
Level
-
Shifting focus on new drug modalities
-
Expansion into emerging markets
Level
-
Frequent and inconvenient intravitreal injection leading to patient non-adherence to treatment
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Aging population and growing prevalence of vision disorders
The aging population and the increasing prevalence of vision disorders are major drivers fueling the growth of the ophthalmology market. As life expectancy rises, age-related conditions such as macular degeneration, glaucoma, and diabetic retinopathy are becoming more widespread. This growing patient pool creates a sustained demand for effective treatments, diagnostics, and preventive solutions. Healthcare systems and pharmaceutical companies are responding with enhanced focus and investment in ophthalmic care innovations.
Restraint: Off-label drug use for various indications
Despite advancements, the market faces restraints due to widespread off-label drug use for various indications. Physicians often prescribe existing drugs outside their approved label to treat unmet ophthalmic needs, which can undermine the adoption of novel therapies. This practice can also pose risks of inconsistent treatment outcomes, adverse effects, and regulatory complications. Consequently, companies face challenges in ensuring market acceptance and reimbursement for newly approved, targeted ophthalmology drugs.
Opportunity: Shifting focus on new drug modalities
The market presents significant opportunities as the focus shifts toward new drug modalities. Advances in gene therapy, biologics, and sustained-release drug delivery systems are creating new frontiers in ophthalmic care. These innovations aim to improve treatment efficacy, safety, and patient compliance while addressing unmet clinical needs. With strong investment in R&D and supportive regulatory pathways, companies can leverage these modalities to differentiate their offerings and capture greater market potential.
Challenge: Frequent and inconvenient intravitreal injection leading to patient non-adherence to treatment
A critical challenge in ophthalmology treatment is the frequent and inconvenient nature of intravitreal injections required for many therapies. Patients often find these invasive procedures uncomfortable, leading to poor adherence and suboptimal treatment outcomes. Non-compliance can compromise drug efficacy and overall disease management, ultimately affecting vision preservation and quality of life. This challenge underscores the urgent need for less invasive, long-acting, or alternative delivery methods to improve patient experience and long-term therapeutic success
Ophthalmology Drugs Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Formulation of preservative-free glaucoma and dry eye disease treatments | Enhances ocular surface tolerance, reduces side effects linked to preservatives, and supports better long-term adherence |
 |
Application of monoclonal antibody therapy for retinal vascular diseases | Improves retinal fluid control, stabilizes vision, and lowers the risk of vision impairment in diabetic retinopathy and AMD |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The ophthalmology drug market ecosystem comprises raw material suppliers (such as Lonza and Merck), drug manufacturers (including Roche, Novartis, and Regeneron), and end users (hospitals, ophthalmic clinics, and patients). Active pharmaceutical ingredients (APIs), biologics, and advanced delivery excipients are formulated into eye drops, intravitreal injections, and sustained-release implants. End users drive demand for improved efficacy, safety, and patient convenience, while manufacturers focus on precision drug development and delivery technologies. Collaboration across the entire value chain, from suppliers to clinicians, is essential for advancing innovation and driving market growth in ophthalmic care.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Ophthalmology Drug Market, By Molecule
In 2024, Aflibercept commanded the largest share of the ophthalmology drug market, driven by its strong adoption for wet age-related macular degeneration (AMD), diabetic macular edema, and retinal vein occlusion. Its proven efficacy, favorable safety profile, and established physician trust made it the preferred anti-VEGF therapy. Extended dosing regimens also improved patient compliance and reduced treatment burden, strengthening its dominance despite competition from newer therapies in the ophthalmic landscape.
Ophthalmology Drug Market, By Modality
In 2024, mAbs and fusion proteins dominated the ophthalmology drugs market, holding the largest share due to their proven efficacy and safety in treating conditions like age-related macular degeneration and diabetic retinopathy. Therapies such as aflibercept, ranibizumab, bevacizumab, and farcical benefit from long patent protection and high treatment costs, while intravitreal injection delivery and supportive reimbursement policies further boost adoption. These factors collectively sustain their market leadership and continued growth..
Ophthalmology Drug Market, By Route of Administration
Intravitreal injection holds the highest share in the ophthalmology drugs market. This method delivers medication directly into the vitreous cavity of the eye, ensuring targeted treatment for retinal diseases such as age-related macular degeneration and diabetic macular edema. Its effectiveness, combined with supportive reimbursement policies and the widespread adoption of therapies like aflibercept and ranibizumab, has made intravitreal injection the preferred and dominant route, surpassing systemic methods like intravenous administration.
Ophthalmology Drug Market, By Indication
In 2024, AMD accounted for the highest share by indication in the ophthalmology drugs market. Driven by the growing elderly population and increasing prevalence of retinal disorders, AMD treatments such as aflibercept, ranibizumab, and bevacizumab dominate the market. The high efficacy of these therapies, along with regular intravitreal administration and strong reimbursement support, ensures widespread adoption. This combination of clinical need and effective treatment options solidifies AMD’s leading market position.
Ophthalmology Drug Market, By End User
Hospitals hold the highest share in the end-user market. This is due to their capacity to provide specialized care, access advanced ophthalmic equipment, and administer treatments such as intravitreal injections under controlled settings. Hospitals also serve as primary centers for managing complex retinal disorders, including age-related macular degeneration and diabetic macular edema, ensuring high patient volume and sustained demand for monoclonal antibodies and fusion protein therapies.
REGION
North America to be fastest-growing region in the global market during the forecast period
North America is projected to hold the highest share and is the fastest-growing region in the ophthalmology drugs market. This dominance is driven by the region’s well-established healthcare infrastructure, the high prevalence of retinal disorders such as age-related macular degeneration, and the strong adoption of advanced biologic therapies, including monoclonal antibodies and fusion proteins. Supportive reimbursement policies, ongoing research, and early adoption of innovative treatment modalities further strengthen North America’s leading position in the market.
Ophthalmology Drugs Market: COMPANY EVALUATION MATRIX
In the ophthalmology therapeutics market matrix, Regeneron Pharmaceuticals (Star) leads with a strong market share and an extensive product portfolio, driven by its monoclonal antibodies and fusion proteins, which are widely adopted for retinal diseases such as AMD and diabetic macular edema. AbbVie (Emerging Leader) is gaining visibility with its innovative biologics and targeted therapies, strengthening its position through niche product offerings and expanding clinical adoption. While Regeneron dominates through scale and proven efficacy, AbbVie shows significant potential to move toward the leaders’ quadrant as demand for advanced ophthalmic treatments continues to rise..
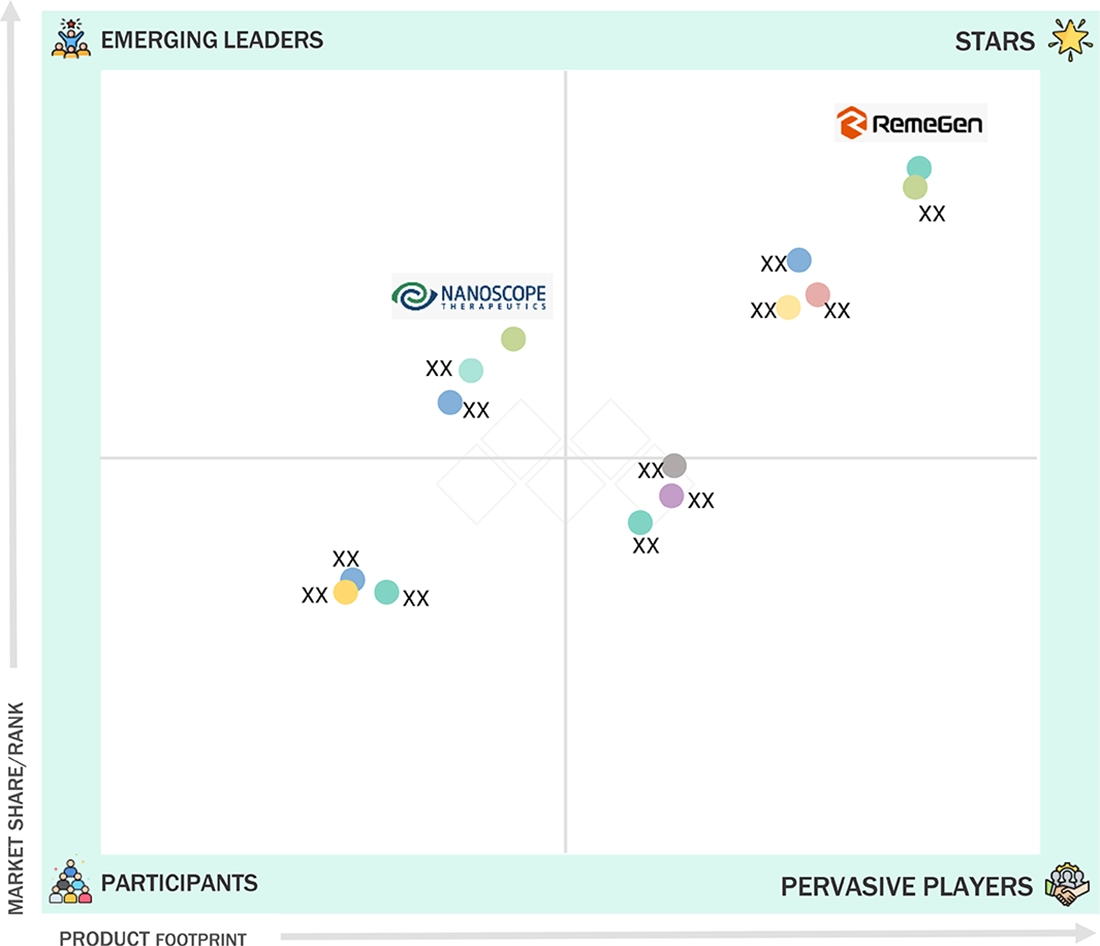
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- F. Hoffmann-La Roche Ltd ( Switzerland)
- Regeneron Pharmaceuticals Inc. (US)
- Novartis AG (Switzerland)
- Bayer AG (Germany)
- Apellis Pharmaceuticals (US)
- Biogen (US)
- Alcon Inc. (US)
- Bausch & Lomb ( Canada)
- AbbVie (US)
- Astellas Pharma Inc. (Japan)
- Formycon AG ( Germany)
- Biocon ( India)
- Sandoz Group AG (Germany)
- ANI Pharmaceuticals, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 18.34 Billion |
| Market Forecast in 2030 (Value) | USD 26.28 Billion |
| Growth Rate | CAGR of 6.1% from 2025-2030 |
| Years Considered | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | North America, Asia Pacific, Europe, Latin America, Middle East and Africa |
WHAT IS IN IT FOR YOU: Ophthalmology Drugs Market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Identifying cost-effective CDMO partners for ophthalmic biologics | Cost benchmarking across sterile fill-finish and cold-chain logistics |
|
| Understand innovation trends in ocular drug delivery | R&D pipeline mapping for sustained-release systems and depot technologies from both companies | Insights on market trends and guide internal innovation strategy |
| Deep dive in regulatory pathways for drug-device combinations | Analysis of global regulatory frameworks for drug-device combinations and biologics | Reduces compliance risks and accelerates approval timelines |
RECENT DEVELOPMENTS
- February 2025 : Teva entered into a strategic collaboration with Klinge Biopharma and Formycon to commercialize FYB203, a biosimilar candidate for Eylea (aflibercept), in most parts of Europe (excluding Italy) and Israel. The biosimilar, branded as AHZANTIVE, is developed by Formycon and has been in-licensed by Klinge for global commercialization.
- December 2024 : Roche received approval from the European Medicines Agency for its Vabysmo (faricimab) 6.0 mg single-dose prefilled syringe (PFS) for the treatment of patients with neovascular or wet age-related macular degeneration (nAMD), diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO). Vabysmo PFS will be the European Union's first and only prefilled syringe containing a bispecific antibody for the treatment of retinal conditions that can cause blindness.
- September 2022 : Outlook Therapeutics, Inc. signed an agreement with Cencora for the commercial launch of Lytenava in the US after the drug was approved by the US Food and Drug Administration (FDA). Cencora will offer third-party logistics services and distribution, as well as medical information and pharmacovigilance services in the US.
Table of Contents

Methodology
This research study extensively used secondary sources, directories, and databases to identify and collect valuable information to analyze the global ophthalmology drugs market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the ophthalmology drugs market. The secondary sources used for this study include US Food and Drug Administration (US FDA), European Medicines Agency (EMA), World Health Organization (WHO), National Center for Biotechnology Information (NCBI), White papers, Company Websites, Interviews with Experts, Annual Reports, SEC Filings, Investor Presentations, and MarketsandMarkets Analysis. These sources also obtained key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
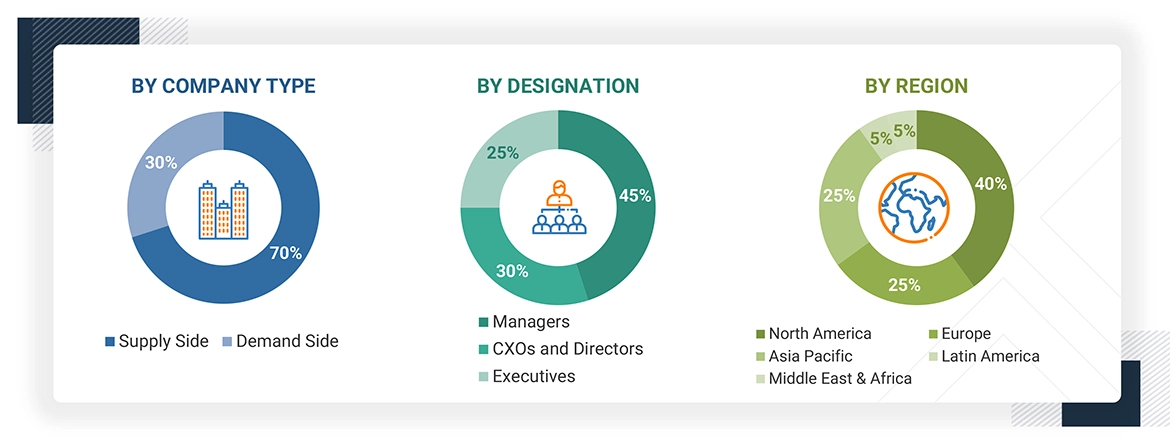
A comprehensive primary research was undertaken following an initial assessment of the global ophthalmology drugs market landscape through secondary research. This involved conducting in-depth interviews with market experts from the demand side, including stakeholders from hospitals, long-term care facilities, and specialty centers. Additionally, interviews were held with key supply-side participants, such as C-suite and senior executives, product managers, and marketing and sales leaders from prominent manufacturers, distributors, and channel partners. The research covered six major geographical regions: North America, Europe, Asia Pacific, Latin America, the Middle East, and Africa. Approximately 70% of the primary interviews were conducted with supply-side participants, while 30% involved demand-side experts. Data collection methods included structured questionnaires, email correspondence, online surveys, personal interviews, and telephonic discussions to understand the market dynamics comprehensively.
The following is a breakdown of the primary respondents:
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
Both bottom-up and top-down approaches were used to estimate and validate the total size of the ophthalmology drugs market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- A list of the major global players operating in the ophthalmology drugs market was generated.
- The revenues generated from their ophthalmology drug products have been determined through annual reports and secondary sources (including paid databases).
- The products were mapped according to the segments of the market. Percentage shares and splits were determined based on the revenue contributed to each segment. This was verified using secondary sources and by industry experts.
- All assumptions, approaches, and individual shares/revenue estimates were validated through expert interviews.
Global Ophthalmology Drugs Market Size: Bottom-up and Top-down Approaches

Data Triangulation
After arriving at the market size from the estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Ophthalmology drugs are specifically designed to treat or prevent eye diseases and disorders. These medications target various parts of the eye, including the cornea, conjunctiva, lens, retina, and optic nerve. The market includes a range of effective therapies across different drug modalities, such as biologics and biosimilars (e.g., anti-VEGF drugs, monoclonal antibodies, oligonucleotides, and peptides), small molecules, gene therapy, and cell therapy. These treatments address various conditions, including age-related macular degeneration (AMD), diabetic retinopathy, diabetic macular edema, retinal vein occlusion, uveitis, and neurotrophic keratitis, among others.
Stakeholders
- Pharmaceutical & biotechnology companies
- Hospitals
- Patient advocacy organizations
- Specialty clinics
- Long-term care facilities
- Academic researchers and government research organizations
- Private research institutes
- Contract manufacturing organizations (CMOs)
- Contract research organizations (CROs)
- Venture capitalists
Report Objectives
- To define, describe, and forecast the ophthalmology drugs market by molecule, modality, route of administration, indication, end user, and region
- To provide detailed information regarding the factors influencing market growth (such as drivers, opportunities, restraints, and challenges)
- To strategically analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall ophthalmology drugs market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To profile the key players in the global ophthalmology drugs market as well as comprehensively analyze their core competencies and market rankings
- To forecast the size of the market segments in North America, Europe, the Asia Pacific, Latin America, the Middle East, and Africa
- To track and analyze competitive developments, such as product launches & approvals, expansions, acquisitions, partnerships, product pipelines, collaborations, and agreements, in the ophthalmology drugs market
Key Questions Addressed by the Report
Who are the key players in the ophthalmology drugs market?
The key players include Regeneron Pharmaceuticals, Inc. (US), F. Hoffmann-La Roche AG (Switzerland), Bayer AG (Germany), and Novartis AG (Switzerland).
Which molecule segment dominates the ophthalmology drugs market?
Aflibercept dominates the market due to its high efficacy and high adoption.
Which indication segment dominated the market in 2024?
The AMD segment dominated in 2024, driven by the rising prevalence of AMD and high adoption of anti-VEGF drugs.
Which regional segment of the ophthalmology drugs market accounted for the largest market share in 2024?
North America held the largest market share in 2024, supported by a strong healthcare ecosystem, high disease burden, and a supportive policy environment.
What is the size and growth rate of the ophthalmology drugs market?
The market is projected to reach USD 26.28 billion by 2030 from USD 19.52 billion in 2025, growing at a CAGR of 6.1%.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Ophthalmology Drugs Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation












Growth opportunities and latent adjacency in Ophthalmology Drugs Market