
Asia Pacific Injectable Drug Delivery Market Size, Growth, Share & Trends Analysis
Asia Pacific Injectable Drug Delivery Market by Product (Device, Autoinjectors, Formulation, Suspensions, Nanoparticles), Site of Administration (Dermal, Organ), Formulation Packaging (Ampoules, Vials), Usage Pattern (Curative, Immunization) - Forecast to 2031




OVERVIEW
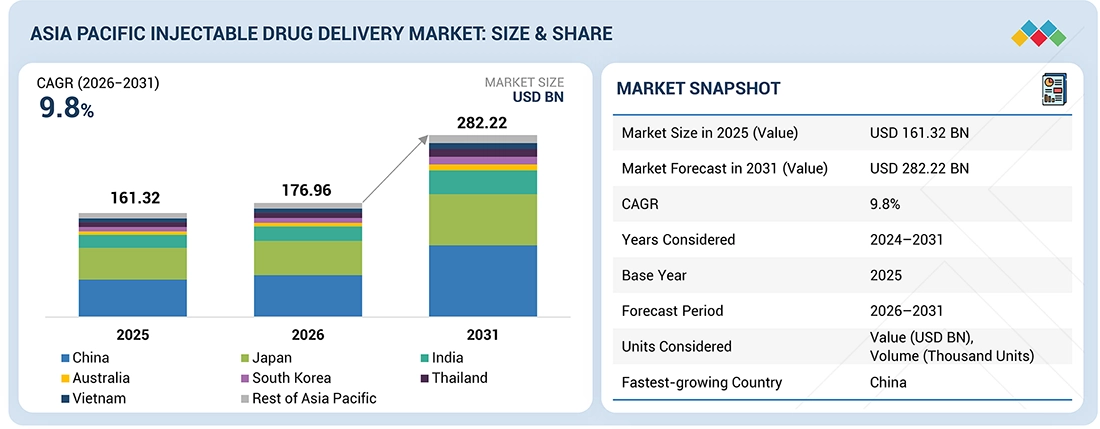
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The Asia Pacific injectable drug delivery market, valued at US$161.32 billion in 2025, stood at US$176.96 billion in 2026 and is projected to advance at a resilient CAGR of 9.8% from 2026 to 2031, culminating in a forecasted valuation of US$282.22 billion by the end of the period. The growth of the Asia Pacific injectable drug delivery market is driven by the increasing incorporation of biologics, biosimilars, and long-acting injectables, alongside a rapidly growing patient population and an escalating burden of chronic diseases across major countries in the region. Patient demand for more patient-centric delivery and self-administration spurs development in formulation science, device engineering capabilities, and scalable sterile manufacturing capacity. The expanding pipeline of oncology, immunology, diabetes, and other specialty therapies, along with the regional adoption of home-based care, integrated digital health delivery systems, and smart connected platforms, is also driving the market.
KEY TAKEAWAYS
-
By CountryChina accounted for the largest share, at 36.1%, in 2025.
-
By ProductBy product, the formulations segment is expected to register the highest CAGR of 10.2%.
-
By Formulation PackagingBy formulation packaging, the ampoules segment is expected to dominate the market with a 43.6% share in 2025.
-
By Therapeutic ApplicationBy therapeutic application, the obesity segment is projected to grow at the fastest rate from 2026 to 2031.
-
By Usage PatternBy usage pattern, the curative care segment accounted for the largest share of 66.0%.
-
By Site of AdministrationBy site of administration, the dermal-based administration is expected to register the highest CAGR of 10.4%
-
By End UserBy end user, the hospitals & clinics segment dominated the market, with a share of 56.4% in 2025.
-
Competitive Landscape - Device Key PlayersBD (US), Terumo Corporation (Japan), and Nipro (Japan) were identified as some of the star players in the Asia Pacific injectable drug delivery market, given their extensive global reach and comprehensive product portfolios.
-
Competitive Landscape - Formulation Key PlayersPfizer Inc. (US), Sanofi (France), and F. Hoffmann-La Roche Ltd (Switzerland) were identified as some of the star players in the Asia Pacific injectable drug delivery market, given their large-scale manufacturing capacity and vast international presence
The growth in the Asia Pacific injectable drug delivery sector stems from the increasing demand for outsourced fill-finish services and device development, as well as the growing emphasis on patient-friendly administration for chronic and specialty conditions. Upcoming innovations such as AI-based formulation tools, aseptic manufacturing automation, smart injectors, and wearable delivery systems, as well as partnerships between pharmaceutical corporations, device developers, and regional CDMOs, are introducing change in the competitive landscape.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The trends affecting the consumer's business in the Asia Pacific injectable drug delivery markets comprise the increased use of biologics and biosimilars, a rapidly aging population, rising cases of chronic diseases, and innovations in drug-device combinations. Pharmaceutical and biotechnology companies are the major users of advanced injectable solutions. The rising need for patient-centric delivery formats has led to an accelerated dependence on integrated drug-device expertise, regional outsourcing partnerships, and innovation-led differentiation in the ecosystem of injectable delivery in the Asia Pacific.
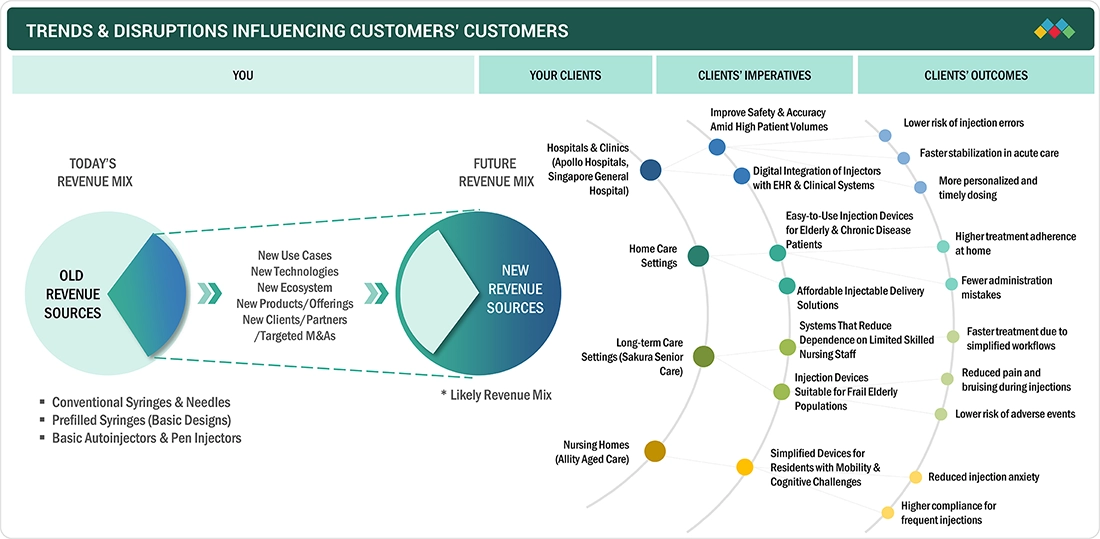
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rapid rise of chronic diseases

-
Increasing demand for self-administration and patient-centric delivery systems
Level
-
High cost of advanced injectable devices and biologic therapies
-
Limited patient awareness and training for advanced injectable devices
Level
-
Expanding biologics & biosimilars pipeline
-
Growing investments in smart injectors, connected devices, and digital health platforms
Level
-
Complex and varying regulatory frameworks
-
Need for scalable sterile manufacturing capacity and aseptic fill-finish expertise
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rapid rise of chronic diseases
The rate of injectable therapies is increasing to meet the rise in incidence rates for chronic diseases, including diabetes, cancer, cardiovascular diseases, and other autoimmune disorders. For most of these chronic diseases, prescription drugs are used, and only potent medicines are administered through injections. As the population grows, more advanced technologies in injectable delivery systems are being developed to enhance therapeutic outcomes in healthcare. This, in turn, serves as a major catalyst for the growth in demand for regional injectable formulations and delivery devices.
Restraint: High cost of advanced injectable devices and biologic therapies
Many advanced injectable devices, especially those for biologics and high-viscosity formulations, are costly to develop and manufacture, making them less affordable for price-sensitive Asia Pacific markets. Biologic drugs also involve high treatment costs, further limiting their wide diffusion. Limitations in reimbursement also constrict patient access in several Asia Pacific countries. Such cost pressures hinder the growth of next-generation injectable technologies.
Opportunity: Expanding biologics & biosimilars pipeline
The Asia Pacific region is emerging as a global hub for biologics and biosimilar development, with significant growth in drugs related to oncology, immunology, and metabolic diseases. Advanced biological therapies require sophisticated and complicated delivery systems by injection, thus creating a strong demand for innovative devices and formulations. Biologics manufacturing is receiving heavy investments from governments and pharmaceutical companies alike. This expanding pipeline poses excellent long-term opportunities for device makers and formulation specialists.
Challenge: Complex and varying regulatory frameworks
The Asia Pacific region is highly heterogeneous, with each country operating under distinct regulatory systems, approval timelines, and pathways for medical device classification. This significantly increases the complexity for companies seeking to launch injectable products across the region. Therefore, the region faces limited harmonization, which increases the compliance burden and development costs. Such fragmented regulation creates hindrances to market entry and obscures commercialization strategies.
Asia Pacific Injectable Drug Delivery Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Operates in the large-scale production of pre-filled syringes, safety syringes, autoinjectors, and combination drugs/devices used largely in hospitals and clinics in Asia Pacific | Promotes a high volume of availability of injectable drugs and safety in injections |
 |
Produces syringes, needles, infusion devices, and insulin patch pumps to provide a wide array of advanced drug delivery systems in Asia Pacific countries | Improves accuracy during dosing| Assists in chronic treatment over a prolonged time frame| Makes patient handling simpler |
 |
Production of glass injection syringes, injection needles, and medical-grade containers | Promotes sterility and prevent contamination of drugs during packaging |
 |
Develops a broad portfolio of sterile injectables, biologic therapies, vaccines, and oncology therapies, which have found widespread usage across Asia Pacific healthcare markets | Provides a reliable way to access high-quality injectables for the treatment of chronic and acute diseases |
 |
Involved in the production of biologic drugs in a wide array of therapy areas, such as immunology, diabetes, oncology, and vaccines | Enhances therapeutic outcomes with stable, high-potency formulations and supports large-scale vaccination programs |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The injectable drug delivery market in the Asia Pacific operates in a dynamic ecosystem involving a broad array of players and stakeholders. The key players in this ecosystem include pharmaceutical firms and device makers providing formulation development, aseptic fill & finish solutions, primary packaging, and combination device engineering for biologic drugs, biosimilars, and complex injectables. They cater to a wide cross-section of customers in biotech, research institutes, and hospitals with a need for advanced delivery technology and scale sterile manufacturing to address an increasing volume of therapeutics. The ecosystem also includes technology partners such as digital health solution providers, automation/robotic firms, and AI-enabled formulation and quality solutions.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
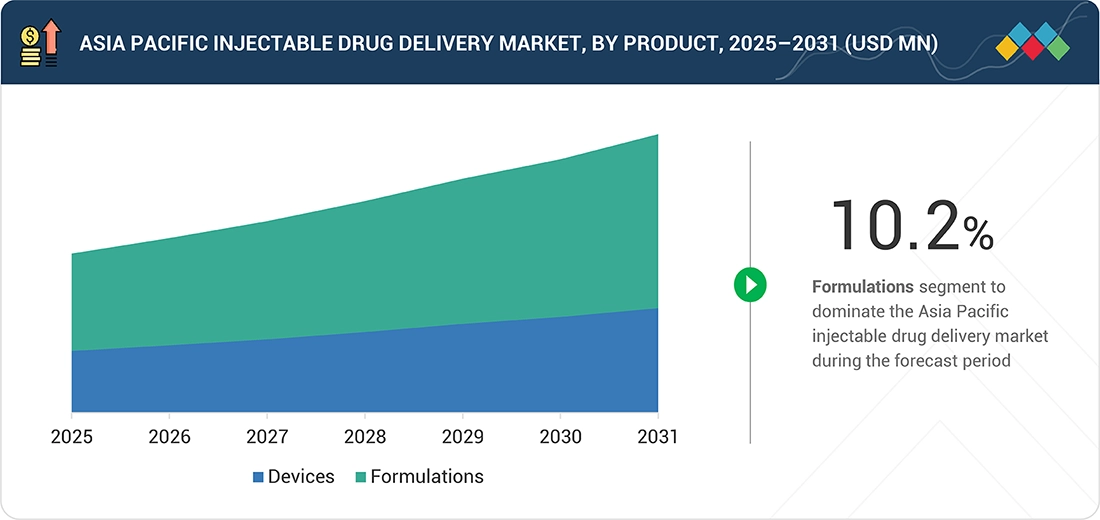
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
APAC Injectable Drug Delivery Market, By Product
As of 2025, formulations accounted for the largest market share in the Asia Pacific injectable drug delivery market. The region is experiencing a growing demand for biologics, biosimilars, vaccines, and chronic therapeutics, wherein advanced formulations are used. Formulations are used in oncology, infectious diseases, diabetes, and immunology drugs. Increased investments in research and a surge in local biologics production capacity in this region are also driving the market.
APAC Injectable Drug Delivery Market, By Formulation Packaging
In 2025, ampoules accounted for the largest market share. They are widely used in hospitals, clinics, and government healthcare projects for vaccination, antibiotics, anesthetics, and acute care injections. They are less expensive to manufacture, highly resistant to chemicals, and compatible with a broad array of drugs, making them ideal in high-volume treatment settings. Well-developed production channels in countries such as China and India, combined with massive vaccination programs, continue to drive a high demand for packaging using ampules.
APAC Injectable Drug Delivery Market, By Therapeutic Application
As of 2025, autoimmune diseases had the largest market share, because the Asia Pacific is witnessing an increase in rheumatoid arthritis, psoriasis, SLE, and inflammatory bowel diseases. These cases are chronic in nature and need long-term injectable biologic and biosimilar drugs. The swift uptake of the latest immunology therapies, better access to specialty physician channels, and rising approvals for targeted biologics and biosimilars are fueling this segment.
APAC Injectable Drug Delivery Market, By Usage Pattern
Curative care had the largest share in 2025, as a considerable portion of injectable therapies within the Asia Pacific is used to treat acute and chronic conditions that require immediate therapeutic intervention. Strong demand for injectables within oncology, diabetes, infectious diseases, autoimmune disorders, and cardiovascular care further solidifies the leading position of curative applications. The use of injectables at hospitals and specialty centers facilitates rapid drug action in diseased conditions. The rise in chronic diseases serves as a significant driving factor for the growth of the segment.
APAC Injectable Drug Delivery Market, By Site of Administration
In 2025, dermal-based accounted for the maximum share since microneedle patches, transdermal systems, and other minimally invasive delivery platforms are being widely adopted in the Asia Pacific. They are easy to handle, leading to reduced pain and increased patient compliance. These technologies facilitate self-administration and are particularly well-suited for vaccines, biologics, and treatments against chronic conditions in markets that strongly prioritize patient comfort and decentralized care. High innovation activity in dermal and needle-free solutions, as well as expanding commercial availability in China, Japan, and South Korea, are also fueling segment growth.
APAC Injectable Drug Delivery Market, By End User
In 2025, hospitals & clinics dominated the end-user market. They remain the foremost treatment centers for acute, chronic, and specialty injectable therapies across the Asia Pacific. They have to manage the largest number of patients with professional facilities, trained staff, and a controlled environment. They also manage complex biologics, oncology injectables, and emergency drugs. The rise in healthcare investments and increase in hospitalization rates further boost the segment growth.
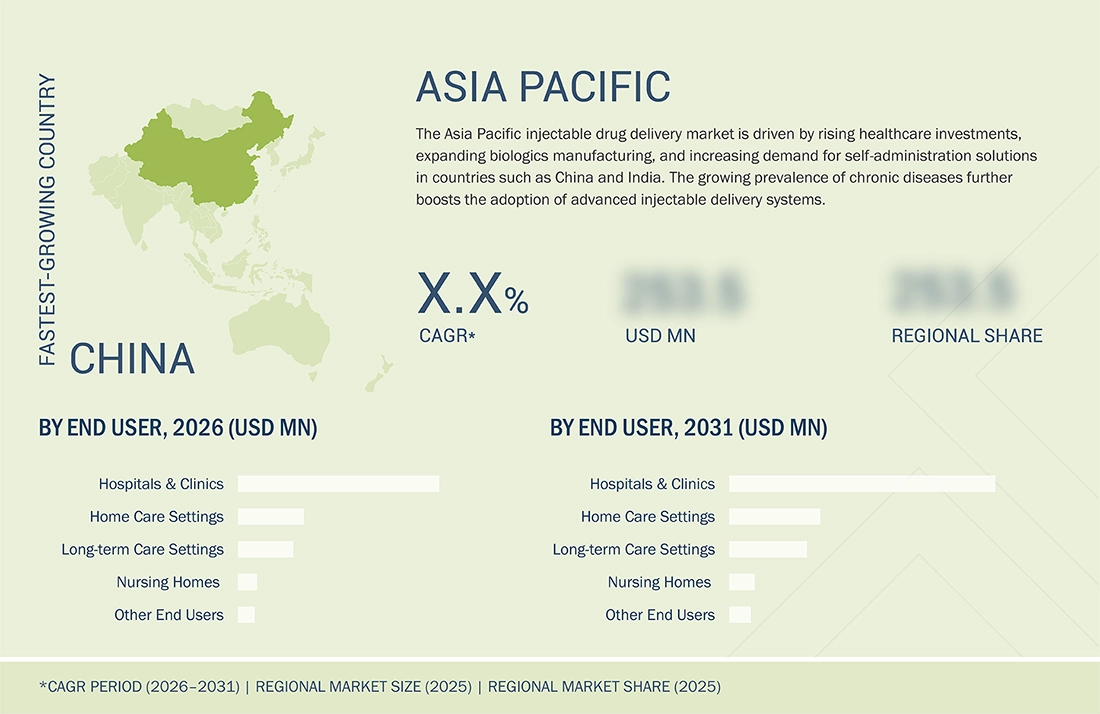
REGION
China to be fastest-growing country in the APAC injectable drug delivery market during forecast period
China is expected to register the highest CAGR during the forecast period. The booming market for biologics & biosimilars, favorable government investment in modernizing healthcare, and a growing prevalence of chronic diseases are also driving the market. The country is moving toward adopting self-administration devices. The country is experiencing faster commercialization of innovative injectable delivery solutions through regulatory reforms.
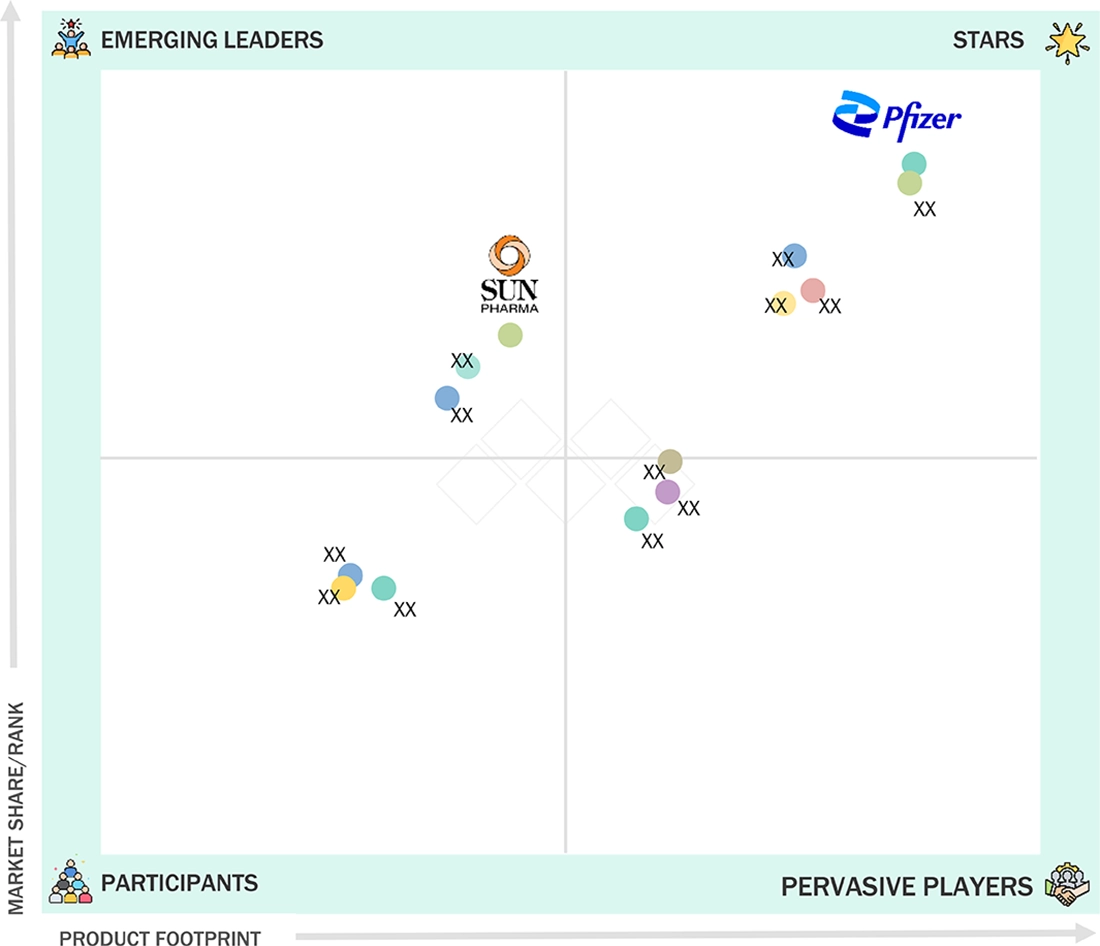
Asia Pacific Injectable Drug Delivery Market: COMPANY EVALUATION MATRIX
In the Asia Pacific injectable drug delivery market matrix, Pfizer Inc. (Star) holds a leading position with a diversified portfolio of biologic drugs, vaccines, and sterile injectables. It has a strong manufacturing scale and heavy investments in R&D. Sun Pharmaceutical Industries Ltd. (Emerging Leader) has a diversified portfolio of complex generics, oncology injectables, and specialty drugs, driven by competitive pricing and increasing investments in sterile product manufacturing.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 161.32 BN |
| Market Forecast in 2031 (Value) | USD 282.22 BN |
| Growth Rate | CAGR of 9.8% from 2026–2031 |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD Billion), Volume (Thousand Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | China, Japan, India, Australia, South Korea, Thailand, Vietnam, New Zealand, Rest of Asia Pacific |
WHAT IS IN IT FOR YOU: Asia Pacific Injectable Drug Delivery Market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis |
|
Enabled understanding of technology maturity, user needs, and product positioning, supporting partner selection, portfolio planning, and investment in next-generation delivery platforms |
| Company Information |
|
Provided insights into partnership potential, biologic–device integration trends, and innovation areas shaping smart injectors and connected delivery systems |
| Geographic Analysis |
|
Supported regional strategy planning by identifying high-growth countries for expansion, localization opportunities, and collaboration hubs across Asia Pacific |
RECENT DEVELOPMENTS
- July 2025 : Terumo Corporation has announced the launch of the Immucise Intradermal Injection System, a convenient method for delivering vaccines and drugs to the dermal layer of the skin.
- June 2025 : Ypsomed AG opened its first dedicated production site in China. This site will help produce 100 million injection devices per year in China.
- October 2024 : BD collaborated with Ypsomed to advance self-injection solutions for high-viscosity biologic drugs, addressing current limitations by enabling the delivery of higher viscosity (>15cP) biologic drugs in an autoinjector format.
Table of Contents

Methodology
This study extensively used both primary and secondary sources. The research process involved studying various factors affecting the industry to identify segmentation types, industry trends, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
This research study involved the usage of comprehensive secondary sources; directories; databases such as Bloomberg Business, Factiva, and Dun & Bradstreet; white papers; annual reports; company house documents; investor presentations; and SEC filings of companies. Secondary research was used to identify and collect information useful for an extensive, technical, market-oriented, and commercial study of the asia pacific injectable drug delivery market. It was also used to identify key players in the market and classify and segment the industry based on trends to the most detailed level. Additionally, significant developments related to market and technology perspectives were noted. A database of the primary industry leaders was also created using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side include project/sales/marketing/business development managers, presidents, CEOs, vice presidents, chairpersons, chief operating officers, chief strategy officers, directors, chief information officers, and chief medical information officers related to the asia pacific injectable drug delivery markets. Primary sources from the demand side include healthcare professionals from hospitals, nursing care facilities, long-term health centers, and ambulatory surgical centers.
To know about the assumptions considered for the study, download the pdf brochure
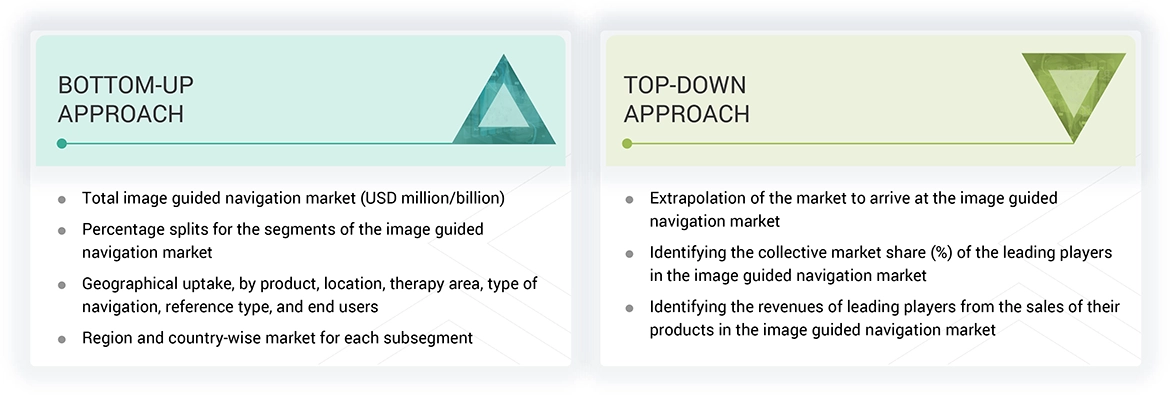
Market Size Estimation
The total size of the asia pacific injectable drug delivery market was determined after data triangulation from three approaches, as mentioned below. After each approach, the weighted average of the three approaches was taken based on the level of assumptions used in each approach.
Data Triangulation
After arriving at the market size, the total market was divided into several segments and subsegments. Data triangulation and market breakdown procedures were employed wherever applicable to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments.
Market Definition
The asia pacific injectable drug delivery market encompasses products, technologies, and services involved in the administration of drugs through injections. It includes a broad range of devices such as conventional syringes, auto-injectors, pen injectors, prefilled syringes, and wearable injectors, as well as various formulations like solutions, suspensions, and emulsions specifically designed for injections. This market addresses the delivery of biologics, vaccines, insulin, cancer therapies, and other treatments requiring precise and rapid drug absorption. It caters to diverse settings, including hospitals, clinics, home care, and ambulatory centers.
Stakeholders
- Injectable Drug and Device Manufacturing Companies
- Pharmaceutical & Injectable Drug Manufacturing Companies
- Healthcare Institutions (Hospitals & Outpatient Clinics)
- Distributors and Suppliers of Injectable Drugs & Devices
- Research Institutes
- Health Insurance Payers
- Market Research and Consulting Firms
Report Objectives
- To define, describe, segment, and forecast the asia pacific injectable drug delivery market by product, therapeutic application, usage pattern, site of administration, end user, and region
- To provide detailed information about the factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall asia pacific injectable drug delivery market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of the asia pacific injectable drug delivery market in six main regions (along with their respective key countries), namely, North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and GCC countries
- To profile the key players in the asia pacific injectable drug delivery market and comprehensively analyze their core competencies and market shares
- To track and analyze competitive developments such as acquisitions, product launches, expansions, collaborations, agreements, and partnerships
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific Injectable Drug Delivery Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Asia Pacific Injectable Drug Delivery Market