2
RESEARCH METHODOLOGY
43
5
MARKET OVERVIEW
Booming cell therapy market driven by innovation in gene editing and regulatory fast-tracking.
64
5.2.1.1
INCREASED FUNDING AND INVESTMENT IN CELL THERAPY
5.2.1.2
ADVANCEMENTS IN GENE EDITING TECHNOLOGIES
5.2.1.3
DEVELOPMENT OF AUTOMATED CELL PROCESSING SYSTEMS
5.2.1.4
REGULATORY SUPPORT FOR FAST-TRACKING THERAPIES
5.2.2.1
MANUFACTURING AND LOGISTICAL COMPLEXITY
5.2.3.1
PERSONALIZED AND OFF-THE-SHELF CELL THERAPIES
5.2.3.2
INTEGRATION WITH DIGITAL TECHNOLOGIES SUCH AS AI & ML
5.2.4.1
SUPPLY CHAIN & COLD CHAIN MANAGEMENT AND MANUFACTURING SCALABILITY & QUALITY CONTROL
5.4.1.1
MAGNETIC-ACTIVATED CELL SORTING
5.4.1.3
GENE EDITING TECHNOLOGIES
5.4.1.4
BIOPROCESSING TECHNOLOGIES
5.4.2
COMPLEMENTARY TECHNOLOGIES
5.4.2.1
ARTIFICIAL INTELLIGENCE AND MACHINE LEARNING
5.4.2.2
BIOMATERIALS AND SCAFFOLDING
5.4.2.3
AUTOMATION AND SCALABILITY
5.4.3
ADJACENT TECHNOLOGIES
5.4.3.2
IMMUNOTHERAPY PLATFORMS
5.5.1
AVERAGE SELLING PRICE TREND OF CELL THERAPY PRODUCTS, BY KEY PLAYER, 2022–2024
5.5.2
AVERAGE SELLING PRICE TREND OF CELL THERAPY EQUIPMENT, BY REGION, 2022–2024
5.8.1
IMPORT DATA FOR MACHINES AND APPARATUS FOR ELECTROPLATING, ELECTROLYSIS, OR ELECTROPHORESIS (HS CODE: 854330)
5.8.2
EXPORT DATA FOR MACHINES AND APPARATUS FOR ELECTROPLATING, ELECTROLYSIS, OR ELECTROPHORESIS (HS CODE: 854330)
5.9
KEY CONFERENCES AND EVENTS, 2025–2026
5.10
REGULATORY LANDSCAPE
5.10.1
REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
5.10.2
REGULATORY FRAMEWORK
5.10.2.4
REST OF THE WORLD
5.11
PORTER’S FIVE FORCES ANALYSIS
5.11.1
THREAT OF NEW ENTRANTS
5.11.2
THREAT OF SUBSTITUTES
5.11.3
BARGAINING POWER OF BUYERS
5.11.4
BARGAINING POWER OF SUPPLIERS
5.11.5
INTENSITY OF COMPETITIVE RIVALRY
5.12
KEY STAKEHOLDERS AND BUYING CRITERIA
5.13
INVESTMENT & FUNDING SCENARIO
5.14
TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESSES
5.15
IMPACT OF AI/GEN AI ON CELL THERAPY TECHNOLOGIES MARKET
6
CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT
Market Size & Growth Rate Forecast Analysis to 2029 in USD Million | 67 Data Tables
98
6.2
MEDIA, SERA, AND REAGENTS
6.2.1
IMPORTANCE OF MEDIA, SERA, AND REAGENTS IN CELL PROCESSING TO DRIVE MARKET
6.3
CELL THERAPY EQUIPMENT
6.3.1
CELL PROCESSING EQUIPMENT
6.3.1.1
DEVELOPMENT OF AUTOMATED AND CLOSED-SYSTEM CELL PROCESSING EQUIPMENT TO ACCELERATE SEGMENT GROWTH
6.3.2
SINGLE-USE EQUIPMENT
6.3.2.1
INCREASING FUNDING FOR STEM CELL THERAPY TO DRIVE MARKET
6.3.3
OTHER CELL THERAPY EQUIPMENT
6.4.1
RISING DEMAND FOR CELL THERAPIES TO SUPPORT MARKET GROWTH
6.5.1
RISING R&D AND INVESTMENTS IN CELL-BASED THERAPIES TO DRIVE DEMAND
6.6
CELL ENGINEERING PRODUCTS
6.6.1
AVAILABILITY OF WIDE RANGE OF CELL ENGINEERING PRODUCTS TO SUPPORT MARKET
7
CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS
Market Size & Growth Rate Forecast Analysis to 2029 in USD Million | 65 Data Tables
130
7.2.1.1
RISING DEMAND FOR PERSONALIZED AND REGENERATIVE THERAPIES TO DRIVE MARKET GROWTH
7.2.2.1
RISING NEED FOR SCALABLE, EFFICIENT ISOLATION TECHNOLOGIES TO DRIVE MARKET
7.2.3
CELL CHARACTERIZATION
7.2.3.1
INCREASING DEMAND FOR HIGH-QUALITY, SAFE, AND EFFECTIVE CELL-BASED THERAPIES TO PROPEL GROWTH
7.2.4.1
ADVANCEMENTS IN CELL COLLECTION TECHNOLOGIES TO DRIVE MARKET
7.3.1
ADVANCEMENT IN CRYOPRESERVATION TECHNOLOGIES TO DRIVE GROWTH
7.4
PROCESS MONITORING & QUALITY CONTROL
7.4.1
INCREASING DEMAND FOR AUTOMATED QUALITY CONTROL IN CELL THERAPY PRODUCTION TO DRIVE MARKET
7.5.1
INCREASING DEMAND FOR AUTOMATED, EFFICIENT, AND SCALABLE SOLUTIONS TO DRIVE MARKET GROWTH
7.6.1
INCREASED FOCUS ON TRANSPORTATION AND STORAGE TO DRIVE MARKET GROWTH
8
CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE
Market Size & Growth Rate Forecast Analysis to 2029 in USD Million | 22 Data Tables
163
8.2.1
RISING RESEARCH ON CAR T-CELL THERAPY TO ACCELERATE GROWTH
8.3.1
INCREASING FUNDING FOR STEM CELL RESEARCH TO PROPEL MARKET
9
CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION
Market Size & Growth Rate Forecast Analysis to 2029 in USD Million | 36 Data Tables
176
9.2.1
RISING RESEARCH ON CAR T-CELL THERAPY TO ACCELERATE SEGMENT GROWTH
9.3
CARDIOVASCULAR DISEASE
9.3.1
INCREASING FOCUS ON INNOVATIVE CELL THERAPY APPROACHES TO DRIVE MARKET
9.4.1
INCREASING FOCUS ON STEM CELL THERAPY FOR TISSUE REGENERATION TO DRIVE MARKET
9.5.1
INCREASING FUNDING FOR STEM CELL RESEARCH TO SUPPORT MARKET GROWTH
10
CELL THERAPY TECHNOLOGIES MARKET, BY END USER
Market Size & Growth Rate Forecast Analysis to 2029 in USD Million | 29 Data Tables
196
10.2
BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
10.2.1
INCREASING ADOPTION OF INORGANIC GROWTH STRATEGIES BY BIOPHARMACEUTICAL COMPANIES TO DRIVE MARKET
10.3.1
FOCUS ON OUTSOURCING TO DRIVE SEGMENT GROWTH
10.4.1
RISING RESEARCH ACTIVITY TO SUPPORT MARKET GROWTH
10.5.1
INCREASING DEMAND FOR STANDARDIZED CELL LINES TO SUPPORT MARKET GROWTH
11
CELL THERAPY TECHNOLOGIES MARKET, BY REGION
Comprehensive coverage of 7 Regions with country-level deep-dive of 16 Countries | 209 Data Tables.
213
11.2.1
MACROECONOMIC OUTLOOK FOR NORTH AMERICA
11.2.2.1
US DOMINATES NORTH AMERICAN CELL THERAPY TECHNOLOGIES MARKET
11.2.3.1
INCREASING GOVERNMENT FUNDING TO DRIVE ADOPTION OF CELL THERAPY INSTRUMENTS
11.3.1
MACROECONOMIC OUTLOOK FOR EUROPE
11.3.2.1
GERMANY TO HOLD LARGEST MARKET SHARE
11.3.3.1
FAVORABLE FUNDING & INVESTMENT SCENARIO TO DRIVE MARKET GROWTH
11.3.4.1
AVAILABILITY OF GOVERNMENT & PRIVATE FUNDING TO SUPPORT MARKET GROWTH
11.3.5.1
INCREASING R&D, PUBLIC & PRIVATE INVESTMENT, AND SUPPORT TO OFFER GROWTH OPPORTUNITIES
11.3.6.1
ADVANCEMENT IN PERSONALIZED MEDICINE TO STIMULATE MARKET
11.4.1
MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
11.4.2.1
INCREASING R&D EXPENDITURE TO DRIVE MARKET GROWTH
11.4.3.1
STRONG AVAILABILITY OF FUNDING TO SUPPORT MARKET GROWTH
11.4.4.1
RISING FUNDING PROGRAMS TO DRIVE MARKET
11.4.5.1
INCREASING GOVERNMENT FUNDING & INITIATIVES TO DRIVE MARKET GROWTH
11.4.6.1
ALLIANCES & INVESTMENTS IN RESEARCH TO DRIVE MARKET
11.4.7
REST OF ASIA PACIFIC
11.5.1
MACROECONOMIC OUTLOOK FOR LATIN AMERICA
11.5.2.1
BRAZIL TO DOMINATE LATIN AMERICAN MARKET TILL 2029
11.5.3.1
STRONG PHARMACEUTICAL INDUSTRY AND INCREASED GOVERNMENT SUPPORT TO AUGMENT MARKET
11.5.4
REST OF LATIN AMERICA
11.6.1
MACROECONOMIC OUTLOOK FOR MIDDLE EAST
11.6.2.1.1
INCREASING GOVERNMENT INVESTMENTS IN HEALTHCARE AND GROWING PHARMACEUTICALS INDUSTRY TO SUPPORT MARKET
11.6.2.2.1
GROWING R&D EXPENDITURE & WELL-ESTABLISHED CLASS INFRASTRUCTURE TO DRIVE MARKET
11.6.2.3
REST OF GCC COUNTRIES
11.6.3
REST OF MIDDLE EAST
11.7.1
MACROECONOMIC OUTLOOK FOR AFRICA
12
COMPETITIVE LANDSCAPE
Discover strategic insights and market positions of key players in cell therapy technologies.
329
12.2
KEY PLAYER STRATEGIES/RIGHT TO WIN
12.2.1
OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN CELL THERAPY TECHNOLOGIES MARKET
12.4
MARKET SHARE ANALYSIS
12.5
COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023
12.5.5
COMPANY FOOTPRINT: KEY PLAYERS, 2023
12.5.5.1
COMPANY FOOTPRINT
12.5.5.2
REGION FOOTPRINT
12.5.5.3
PRODUCT FOOTPRINT
12.5.5.4
PROCESS FOOTPRINT
12.5.5.5
CELL TYPE FOOTPRINT
12.5.5.6
APPLICATION FOOTPRINT
12.6
COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
12.6.1
PROGRESSIVE COMPANIES
12.6.2
RESPONSIVE COMPANIES
12.6.5
COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023
12.7
COMPANY VALUATION & FINANCIAL METRICS
12.8
BRAND/PRODUCT COMPARISON
12.9
COMPETITIVE SCENARIO
13
COMPANY PROFILES
In-depth Company Profiles of Leading Market Players with detailed Business Overview, Product and Service Portfolio, Recent Developments, and Unique Analyst Perspective (MnM View)
350
13.1.1
DANAHER CORPORATION
13.1.1.1
BUSINESS OVERVIEW
13.1.1.2
PRODUCTS OFFERED
13.1.1.3
RECENT DEVELOPMENTS
13.1.1.3.1
PRODUCT LAUNCHES
13.1.1.4.2
STRATEGIC CHOICES
13.1.1.4.3
WEAKNESSES AND COMPETITIVE THREATS
13.1.3
THERMO FISHER SCIENTIFIC INC.
13.1.6
AGILENT TECHNOLOGIES, INC.
13.1.8
FRESENIUS SE & CO. KGAA
13.1.9
BECTON, DICKINSON AND COMPANY
13.1.10
CORNING INCORPORATED
13.1.11
TERUMO CORPORATION
13.1.15
STEMCELL TECHNOLOGIES
13.2.1
OXFORD BIOMEDICA PLC
13.2.4
L7 INFORMATICS, INC.
13.2.6
REPLIGEN CORPORATION
13.2.8
ORIGEN BIOMEDICAL, INC.
13.2.9
IXCELLS BIOTECHNOLOGIES
14.2
KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
14.3
CUSTOMIZATION OPTIONS
TABLE 1
IMPACT ANALYSIS OF SUPPLY-SIDE AND DEMAND-SIDE FACTORS
TABLE 2
CELL THERAPY TECHNOLOGIES MARKET: RISK ANALYSIS
TABLE 3
CELL THERAPY TECHNOLOGIES MARKET: KEY PRODUCT PROVIDERS
TABLE 4
CELL THERAPY TECHNOLOGIES MARKET: KEY END USERS
TABLE 5
CELL THERAPY TECHNOLOGIES MARKET: REGULATORY BODIES
TABLE 6
AVERAGE SELLING PRICE TREND OF CELL THERAPY PRODUCTS, BY KEY PLAYER, 2022–2024
TABLE 7
AVERAGE SELLING PRICE TREND OF CELL THERAPY EQUIPMENT, BY REGION, 2022–2024
TABLE 8
KEY PATENTS IN CELL THERAPY TECHNOLOGIES MARKET, 2021–2024
TABLE 9
IMPORT VOLUME FOR HS CODE 854330, 2019–2023 (UNITS)
TABLE 10
EXPORT VOLUME FOR HS CODE 854330, 2019–2023 (UNITS)
TABLE 11
CELL THERAPY TECHNOLOGIES MARKET: DETAILED LIST OF CONFERENCES & EVENTS
TABLE 12
NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 13
EUROPE: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 14
ASIA PACIFIC: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 15
REST OF THE WORLD: LIST OF REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
TABLE 16
CELL THERAPY TECHNOLOGIES MARKET: PORTER’S FIVE FORCES ANALYSIS
TABLE 17
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR TOP FOUR END USERS
TABLE 18
KEY BUYING CRITERIA FOR CELL THERAPY TECHNOLOGIES PRODUCTS, BY END USER
TABLE 19
CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 20
CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 21
NORTH AMERICA: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 22
EUROPE: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 23
ASIA PACIFIC: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 24
LATIN AMERICA: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 25
MIDDLE EAST: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 26
GCC COUNTRIES: CELL THERAPY MEDIA, SERA, AND REAGENTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 27
CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 28
CELL THERAPY EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 29
NORTH AMERICA: CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 30
EUROPE: CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 31
ASIA PACIFIC: CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 32
LATIN AMERICA: CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 33
MIDDLE EAST: CELL THERAPY EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 34
GCC COUNTRIES: CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 35
CELL PROCESSING EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 36
NORTH AMERICA: CELL PROCESSING EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 37
EUROPE: CELL PROCESSING EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 38
ASIA PACIFIC: CELL PROCESSING EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 39
LATIN AMERICA: CELL PROCESSING EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 40
MIDDLE EAST: CELL PROCESSING EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 41
GCC COUNTRIES: CELL PROCESSING EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 42
SINGLE-USE EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 43
NORTH AMERICA: SINGLE-USE EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 44
EUROPE: SINGLE-USE EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 45
ASIA PACIFIC: SINGLE-USE EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 46
LATIN AMERICA: SINGLE-USE EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 47
MIDDLE EAST: SINGLE-USE EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 48
GCC COUNTRIES: SINGLE-USE EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 49
OTHER CELL THERAPY EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 50
NORTH AMERICA: OTHER CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 51
EUROPE: OTHER CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 52
ASIA PACIFIC: OTHER CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 53
LATIN AMERICA: OTHER CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 54
MIDDLE EAST: OTHER CELL THERAPY EQUIPMENT MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 55
GCC COUNTRIES: OTHER CELL THERAPY EQUIPMENT MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 56
CELL THERAPY SYSTEMS & SOFTWARE, BY COMPANY
TABLE 57
CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 58
NORTH AMERICA: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 59
EUROPE: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 60
ASIA PACIFIC: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 61
LATIN AMERICA: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 62
MIDDLE EAST: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 63
GCC COUNTRIES: CELL THERAPY SYSTEMS & SOFTWARE MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 64
CELL CULTURE VESSELS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 65
NORTH AMERICA: CELL CULTURE VESSELS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 66
EUROPE: CELL CULTURE VESSELS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 67
ASIA PACIFIC: CELL CULTURE VESSELS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 68
LATIN AMERICA: CELL CULTURE VESSELS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 69
MIDDLE EAST: CELL CULTURE VESSELS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 70
GCC COUNTRIES: CELL CULTURE VESSELS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 71
CELL ENGINEERING PRODUCTS OFFERED, BY COMPANY
TABLE 72
CELL ENGINEERING PRODUCTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 73
NORTH AMERICA: CELL ENGINEERING PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 74
EUROPE: CELL ENGINEERING PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 75
ASIA PACIFIC: CELL ENGINEERING PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 76
LATIN AMERICA: CELL ENGINEERING PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 77
MIDDLE EAST: CELL ENGINEERING PRODUCTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 78
GCC COUNTRIES: CELL ENGINEERING PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 79
OTHER CELL THERAPY PRODUCTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 80
NORTH AMERICA: OTHER CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 81
EUROPE: OTHER CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 82
ASIA PACIFIC: OTHER CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 83
LATIN AMERICA: OTHER CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 84
MIDDLE EAST: OTHER CELL THERAPY PRODUCTS MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 85
GCC COUNTRIES: OTHER CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 86
CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 87
CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 88
CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY REGION, 2022–2029 (USD MILLION)
TABLE 89
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 90
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 91
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 92
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 93
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY REGION, 2022–2029 (USD MILLION)
TABLE 94
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 95
CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY REGION, 2022–2029 (USD MILLION)
TABLE 96
CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 97
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 98
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 99
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 100
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY REGION, 2022–2029 (USD MILLION)
TABLE 101
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL EXPANSION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 102
CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 103
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 104
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 105
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 106
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 107
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 108
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL ISOLATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 109
CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 110
CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 111
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 112
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 113
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 114
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 115
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL CHARACTERIZATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 116
CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY REGION, 2022–2029 (USD MILLION)
TABLE 117
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 118
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 119
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 120
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 121
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY REGION, 2022–2029 (USD MILLION)
TABLE 122
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL COLLECTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 123
CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 124
CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 125
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 126
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 127
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 128
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY REGION, 2022–2029 (USD MILLION)
TABLE 129
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PRESERVATION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 130
CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY REGION, 2022–2029 (USD MILLION)
TABLE 131
CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 132
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 133
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 134
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 135
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY REGION, 2022–2029 (USD MILLION)
TABLE 136
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR PROCESS MONITORING & QUALITY CONTROL, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 137
CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY REGION, 2022–2029 (USD MILLION)
TABLE 138
CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 139
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 140
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 141
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 142
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY REGION, 2022–2029 (USD MILLION)
TABLE 143
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL HANDLING, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 144
CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY REGION, 2022–2029 (USD MILLION)
TABLE 145
CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 146
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 147
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 148
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 149
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY REGION, 2022–2029 (USD MILLION)
TABLE 150
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL DISTRIBUTION, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 151
CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 152
T-CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 153
NORTH AMERICA: T-CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 154
EUROPE: T-CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 155
ASIA PACIFIC: T-CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 156
LATIN AMERICA: T-CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 157
MIDDLE EAST: T-CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 158
GCC COUNTRIES: T-CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 159
STEM CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 160
NORTH AMERICA: STEM CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 161
EUROPE: STEM CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 162
ASIA PACIFIC: STEM CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 163
LATIN AMERICA: STEM CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 164
MIDDLE EAST: STEM CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 165
GCC COUNTRIES: STEM CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 166
OTHER CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 167
NORTH AMERICA: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 168
EUROPE: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 169
ASIA PACIFIC: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 170
LATIN AMERICA: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 171
MIDDLE EAST: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 172
GCC COUNTRIES: OTHER CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 173
CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 174
CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY REGION, 2022–2029 (USD MILLION)
TABLE 175
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 176
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 177
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 178
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 179
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY REGION, 2022–2029 (USD MILLION)
TABLE 180
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CANCER, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 181
CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY REGION, 2022–2029 (USD MILLION)
TABLE 182
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 183
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 184
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 185
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 186
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY REGION, 2022–2029 (USD MILLION)
TABLE 187
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CARDIOVASCULAR DISEASE, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 188
CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY REGION, 2022–2029 (USD MILLION)
TABLE 189
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 190
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 191
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 192
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 193
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY REGION, 2022–2029 (USD MILLION)
TABLE 194
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR ORTHOPEDIC DISORDERS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 195
CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY REGION, 2022–2029 (USD MILLION)
TABLE 196
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 197
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 198
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 199
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 200
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY REGION, 2022–2029 (USD MILLION)
TABLE 201
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR AUTOIMMUNE DISEASES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 202
CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY REGION, 2022–2029 (USD MILLION)
TABLE 203
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 204
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 205
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 206
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 207
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY REGION, 2022–2029 (USD MILLION)
TABLE 208
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR OTHER APPLICATIONS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 209
CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 210
CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2022–2029 (USD MILLION)
TABLE 211
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 212
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 213
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 214
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 215
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2022–2029 (USD MILLION)
TABLE 216
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR BIOPHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 217
CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY REGION, 2022–2029 (USD MILLION)
TABLE 218
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 219
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 220
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 221
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 222
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY REGION, 2022–2029 (USD MILLION)
TABLE 223
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CROS & CMOS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 224
CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY REGION, 2022–2029 (USD MILLION)
TABLE 225
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 226
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 227
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 228
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 229
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY REGION, 2022–2029 (USD MILLION)
TABLE 230
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR RESEARCH INSTITUTES, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 231
CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY REGION, 2022–2029 (USD MILLION)
TABLE 232
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 233
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 234
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 235
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 236
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY REGION, 2022–2029 (USD MILLION)
TABLE 237
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL BANKS, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 238
CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 239
NORTH AMERICA: KEY MACROINDICATORS
TABLE 240
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 241
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 242
NORTH AMERICA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 243
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 244
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 245
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 246
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 247
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 248
US: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 249
US: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 250
US: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 251
US: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 252
US: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 253
US: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 254
US: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 255
CANADA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 256
CANADA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 257
CANADA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 258
CANADA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 259
CANADA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 260
CANADA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 261
CANADA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 262
EUROPE: KEY MACROINDICATORS
TABLE 263
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 264
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 265
EUROPE: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 266
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 267
EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 268
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 269
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 270
EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 271
GERMANY: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 272
GERMANY: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 273
GERMANY: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 274
GERMANY: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 275
GERMANY: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 276
GERMANY: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 277
GERMANY: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 278
UK: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 279
UK: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 280
UK: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 281
UK: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 282
UK: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 283
UK: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 284
UK: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 285
FRANCE: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 286
FRANCE: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 287
FRANCE: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 288
FRANCE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 289
FRANCE: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 290
FRANCE: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 291
FRANCE: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 292
ITALY: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 293
ITALY: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 294
ITALY: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 295
ITALY: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 296
ITALY: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 297
ITALY: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 298
ITALY: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 299
SPAIN: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 300
SPAIN: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 301
SPAIN: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 302
SPAIN: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 303
SPAIN: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 304
SPAIN: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 305
SPAIN: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 306
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 307
REST OF EUROPE: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 308
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 309
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 310
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 311
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 312
REST OF EUROPE: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 313
ASIA PACIFIC: KEY MACROINDICATORS
TABLE 314
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 315
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 316
ASIA PACIFIC: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 317
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 318
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 319
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 320
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 321
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 322
CHINA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 323
CHINA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 324
CHINA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 325
CHINA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 326
CHINA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 327
CHINA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 328
CHINA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 329
JAPAN: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 330
JAPAN: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 331
JAPAN: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 332
JAPAN: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 333
JAPAN: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 334
JAPAN: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 335
JAPAN: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 336
INDIA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 337
INDIA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 338
INDIA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 339
INDIA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 340
INDIA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 341
INDIA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 342
INDIA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 343
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 344
AUSTRALIA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 345
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 346
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 347
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 348
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 349
AUSTRALIA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 350
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 351
SOUTH KOREA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 352
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 353
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 354
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 355
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 356
SOUTH KOREA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 357
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 358
REST OF ASIA PACIFIC: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 359
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 360
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 361
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 362
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 363
REST OF ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 364
LATIN AMERICA: KEY MACROINDICATORS
TABLE 365
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 366
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 367
LATIN AMERICA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 368
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 369
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 370
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 371
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 372
LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 373
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 374
BRAZIL: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 375
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 376
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 377
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 378
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 379
BRAZIL: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 380
MEXICO: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 381
MEXICO: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 382
MEXICO: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 383
MEXICO: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 384
MEXICO: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 385
MEXICO: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 386
MEXICO: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 387
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 388
REST OF LATIN AMERICA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 389
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 390
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 391
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 392
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 393
REST OF LATIN AMERICA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 394
MIDDLE EAST: KEY MACROINDICATORS
TABLE 395
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY REGION, 2022–2029 (USD MILLION)
TABLE 396
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 397
MIDDLE EAST: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 398
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 399
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 400
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 401
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 402
MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 403
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY COUNTRY, 2022–2029 (USD MILLION)
TABLE 404
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 405
GCC COUNTRIES: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 406
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 407
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 408
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 409
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 410
GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 411
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 412
SAUDI ARABIA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 413
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 414
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 415
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 416
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 417
SAUDI ARABIA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 418
UAE: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 419
UAE: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 420
UAE: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 421
UAE: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 422
UAE: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 423
UAE: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 424
UAE: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 425
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 426
REST OF GCC COUNTRIES: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 427
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 428
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 429
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 430
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 431
REST OF GCC COUNTRIES: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 432
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 433
REST OF MIDDLE EAST: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 434
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 435
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 436
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 437
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 438
REST OF MIDDLE EAST: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 439
AFRICA: KEY MACROINDICATORS
TABLE 440
AFRICA: CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2022–2029 (USD MILLION)
TABLE 441
AFRICA: CELL THERAPY EQUIPMENT MARKET, BY TYPE, 2022–2029 (USD MILLION)
TABLE 442
AFRICA: CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2022–2029 (USD MILLION)
TABLE 443
AFRICA: CELL THERAPY TECHNOLOGIES MARKET FOR CELL PROCESSING, BY TYPE, 2022–2029 (USD MILLION)
TABLE 444
AFRICA: CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2022–2029 (USD MILLION)
TABLE 445
AFRICA: CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2022–2029 (USD MILLION)
TABLE 446
AFRICA: CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2022–2029 (USD MILLION)
TABLE 447
OVERVIEW OF STRATEGIES DEPLOYED BY KEY MANUFACTURING COMPANIES, 2021–2023
TABLE 448
CELL THERAPY TECHNOLOGIES MARKET: DEGREE OF COMPETITION
TABLE 449
CELL THERAPY TECHNOLOGIES MARKET: REGION FOOTPRINT
TABLE 450
CELL THERAPY TECHNOLOGIES MARKET: PRODUCT FOOTPRINT
TABLE 451
CELL THERAPY TECHNOLOGIES MARKET: PROCESS FOOTPRINT
TABLE 452
CELL THERAPY TECHNOLOGIES MARKET: CELL TYPE FOOTPRINT
TABLE 453
CELL THERAPY TECHNOLOGIES MARKET: APPLICATION FOOTPRINT
TABLE 454
CELL THERAPY TECHNOLOGIES MARKET: DETAILED LIST OF KEY STARTUPS/SMES
TABLE 455
CELL THERAPY TECHNOLOGIES MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES
TABLE 456
CELL THERAPY TECHNOLOGIES MARKET: PRODUCT LAUNCHES, JANUARY 2021–NOVEMBER 2024
TABLE 457
CELL THERAPY TECHNOLOGIES MARKET: DEALS, JANUARY 2021– NOVEMBER 2024
TABLE 458
CELL THERAPY TECHNOLOGIES MARKET: EXPANSIONS, JANUARY 2021– NOVEMBER 2024
TABLE 459
DANAHER CORPORATION: COMPANY OVERVIEW
TABLE 460
DANAHER CORPORATION: PRODUCTS OFFERED
TABLE 461
DANAHER CORPORATION: PRODUCT LAUNCHES, JANUARY 2021– OCTOBER 2024
TABLE 462
DANAHER CORPORATION: DEALS, JANUARY 2021– OCTOBER 2024
TABLE 463
DANAHER CORPORATION: EXPANSIONS, JANUARY 2021– OCTOBER 2024
TABLE 464
MERCK KGAA: COMPANY OVERVIEW
TABLE 465
MERCK KGAA: PRODUCTS OFFERED
TABLE 466
MERCK KGAA: DEALS, JANUARY 2021–SEPTEMBER 2024
TABLE 467
MERCK KGAA: EXPANSIONS, JANUARY 2021–SEPTEMBER 2024
TABLE 468
THERMO FISHER SCIENTIFIC INC: COMPANY OVERVIEW
TABLE 469
THERMO FISHER SCIENTIFIC INC: PRODUCTS OFFERED
TABLE 470
THERMO FISHER SCIENTIFIC INC: PRODUCT LAUNCHES, JANUARY 2021–SEPTEMBER 2024
TABLE 471
THERMO FISHER SCIENTIFIC INC: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 472
THERMO FISHER SCIENTIFIC INC: EXPANSIONS, JANUARY 2021–OCTOBER 2024
TABLE 473
LONZA: COMPANY OVERVIEW
TABLE 474
LONZA: PRODUCTS OFFERED
TABLE 475
LONZA: PRODUCT LAUNCHES, JANUARY 2021– OCTOBER 2024
TABLE 476
LONZA: DEALS, JANUARY 2021– OCTOBER 2024
TABLE 477
SARTORIUS: COMPANY OVERVIEW
TABLE 478
SARTORIUS AG: PRODUCTS OFFERED
TABLE 479
SARTORIUS AG: PRODUCT LAUNCHES, JANUARY 2021–OCTOBER 2024
TABLE 480
SARTORIUS AG: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 481
SARTORIUS AG: EXPANSIONS, JANUARY 2021– OCTOBER 2024
TABLE 482
AGILENT TECHNOLOGIES: COMPANY OVERVIEW
TABLE 483
AGILENT TECHNOLOGIES: PRODUCTS OFFERED
TABLE 484
AGILENT TECHNOLOGIES: PRODUCT LAUNCHES, JANUARY 2021–OCTOBER 2024
TABLE 485
AGILENT TECHNOLOGIES: DEALS, JANUARY 2021– OCTOBER 2024
TABLE 486
AVANTOR: COMPANY OVERVIEW
TABLE 487
AVANTOR: PRODUCTS OFFERED
TABLE 488
AVANTOR: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 489
FRESENIUS: COMPANY OVERVIEW
TABLE 490
FRESENIUS: PRODUCTS OFFERED
TABLE 491
FRESENIUS: DEALS, JANUARY 2021–SEPTEMBER 2024
TABLE 492
BD: COMPANY OVERVIEW
TABLE 493
BD: PRODUCTS OFFERED
TABLE 494
BD: PRODUCT LAUNCHES, JANUARY 2021– OCTOBER 2024
TABLE 495
CORNING INCORPORATED: COMPANY OVERVIEW
TABLE 496
CORNING INCORPORATED: PRODUCTS OFFERED
TABLE 497
TERUMO CORPORATION: COMPANY OVERVIEW
TABLE 498
TERUMO CORPORATION: PRODUCTS OFFERED
TABLE 499
TERUMO CORPORATION: PRODUCT LAUNCHES, JANUARY 2021–OCTOBER 2024
TABLE 500
TERUMO CORPORATION: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 501
TERUMO CORPORATION: EXPANSIONS, JANUARY 2021–OCTOBER 2024
TABLE 502
BIO-TECHNE: COMPANY OVERVIEW
TABLE 503
BIO-TECHNE: PRODUCTS OFFERED
TABLE 504
BIO-TECHNE: PRODUCT LAUNCHES, JANUARY 2021–OCTOBER 2024
TABLE 505
BIO-TECHNE: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 506
BIO-TECHNE: OTHER DEVELOPMENTS, JANUARY 2021–OCTOBER 2024
TABLE 507
GENSCRIPT: COMPANY OVERVIEW
TABLE 508
GENSCRIPT: PRODUCTS OFFERED
TABLE 509
GENSCRIPT: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 510
MAXCYTE: COMPANY OVERVIEW
TABLE 511
MAXCYTE: PRODUCTS OFFERED
TABLE 512
MAXCYTE: DEALS, JANUARY 2021–OCTOBER 2024
TABLE 513
STEMCELL TECHNOLOGIES: COMPANY OVERVIEW
TABLE 514
STEMCELL TECHNOLOGIES: PRODUCTS OFFERED
TABLE 515
STEMCELL TECHNOLOGIES: PRODUCT LAUNCHES, JANUARY 2021– OCTOBER 2024
TABLE 516
STEMCELL TECHNOLOGIES: DEALS, JANUARY 2021–OCTOBER 2024
FIGURE 1
CELL THERAPY TECHNOLOGIES MARKET SEGMENTATION AND REGIONAL SCOPE
FIGURE 2
CELL THERAPY TECHNOLOGIES MARKET: RESEARCH DESIGN
FIGURE 3
CELL THERAPY TECHNOLOGIES MARKET: BREAKDOWN OF PRIMARY INTERVIEWS
FIGURE 4
CELL THERAPY TECHNOLOGIES MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2023
FIGURE 5
MARKET SIZE ESTIMATION APPROACH 1 (COMPANY REVENUE ANALYSIS-BASED ESTIMATION), 2023
FIGURE 6
ILLUSTRATIVE EXAMPLE OF DANAHER CORPORATION: REVENUE SHARE ANALYSIS (2023)
FIGURE 7
MARKET SIZE VALIDATION FROM PRIMARY SOURCES
FIGURE 8
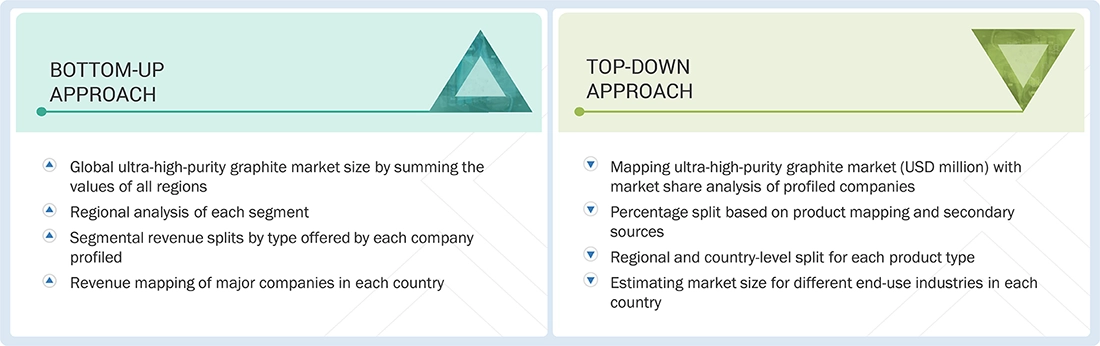
MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
FIGURE 9
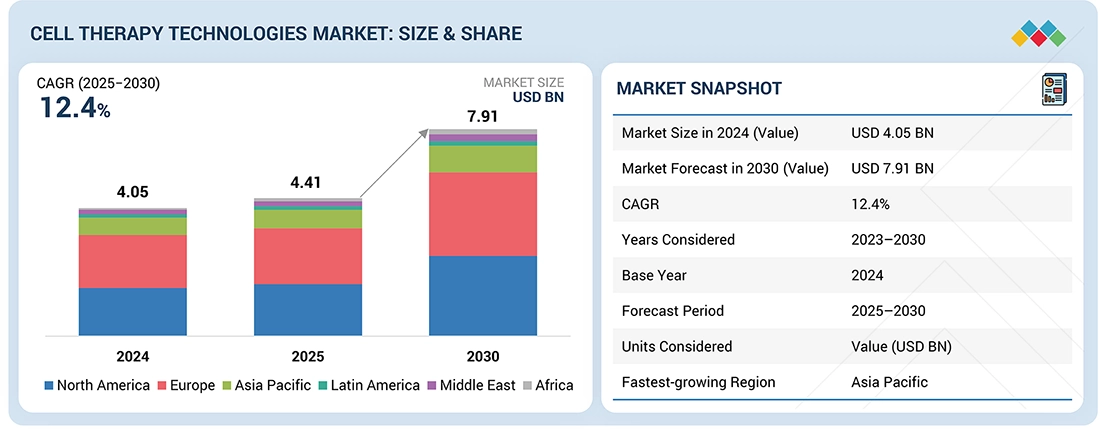
CELL THERAPY TECHNOLOGIES MARKET: CAGR PROJECTIONS
FIGURE 10
DATA TRIANGULATION METHODOLOGY
FIGURE 11
CELL THERAPY TECHNOLOGIES MARKET, BY PRODUCT, 2024 VS. 2029 (USD MILLION)
FIGURE 12
CELL THERAPY TECHNOLOGIES MARKET, BY PROCESS, 2024 VS. 2029 (USD MILLION)
FIGURE 13
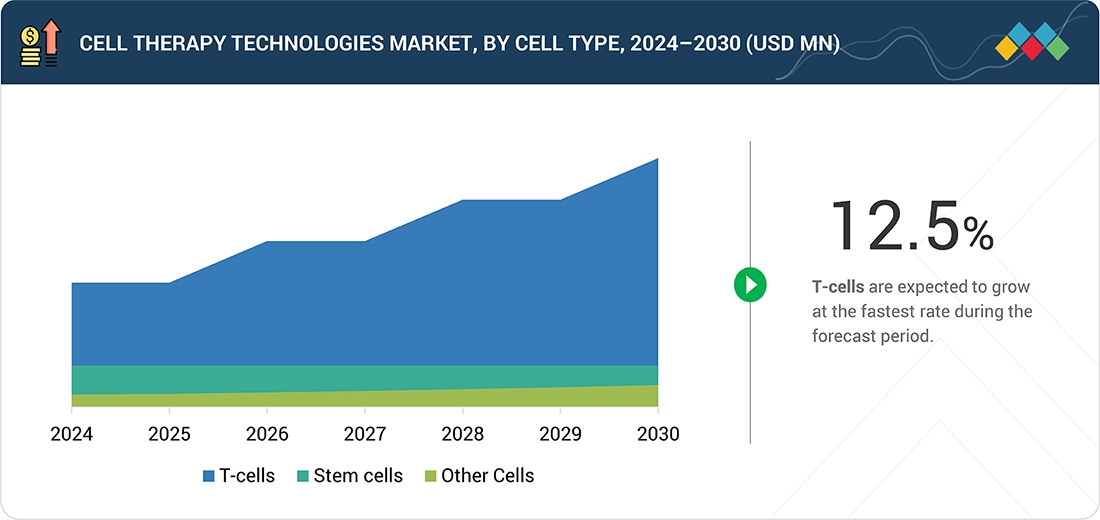
CELL THERAPY TECHNOLOGIES MARKET, BY CELL TYPE, 2024 VS. 2029 (USD MILLION)
FIGURE 14
CELL THERAPY TECHNOLOGIES MARKET, BY APPLICATION, 2024 VS. 2029 (USD MILLION)
FIGURE 15
CELL THERAPY TECHNOLOGIES MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
FIGURE 16
GEOGRAPHICAL SNAPSHOT OF CELL THERAPY TECHNOLOGIES MARKET (2024-2029)
FIGURE 17
INCREASED FUNDING & INVESTMENT IN CELL THERAPY AND ADVANCEMENTS IN GENE EDITING TECHNOLOGIES TO DRIVE MARKET
FIGURE 18
US ACCOUNTED FOR LARGEST MARKET SHARE IN 2023
FIGURE 19
MEDIA, SERA, AND REAGENTS TO DOMINATE MARKET DURING FORECAST PERIOD
FIGURE 20
APAC COUNTRIES TO REGISTER HIGHEST CAGR DURING FORECAST PERIOD
FIGURE 21
CELL THERAPY TECHNOLOGIES MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
FIGURE 22
CELL THERAPY TECHNOLOGIES MARKET: ECOSYSTEM
FIGURE 23
AVERAGE SELLING PRICE TREND OF CELL THERAPY EQUIPMENT, 2024 (USD THOUSAND)
FIGURE 24
CELL THERAPY TECHNOLOGIES MARKET: VALUE CHAIN ANALYSIS
FIGURE 25
CELL THERAPY TECHNOLOGIES MARKET: OVERVIEW OF PATENTING ACTIVITY
FIGURE 26
CELL THERAPY TECHNOLOGIES MARKET: PORTER’S FIVE FORCES ANALYSIS
FIGURE 27
INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS
FIGURE 28
KEY BUYING CRITERIA FOR TOP FOUR END USERS
FIGURE 29
GOVERNMENT FUNDING FOR R&D FROM 2019–2024
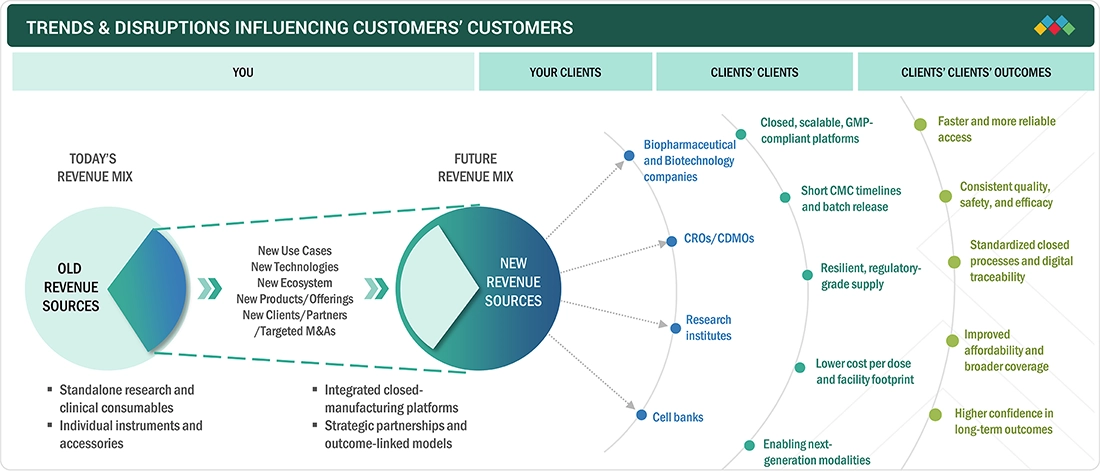
FIGURE 30
TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
FIGURE 31
CELL THERAPY TECHNOLOGIES MARKET: IMPACT OF AI
FIGURE 32
NORTH AMERICA: CELL THERAPY TECHNOLOGIES MARKET SNAPSHOT
FIGURE 33
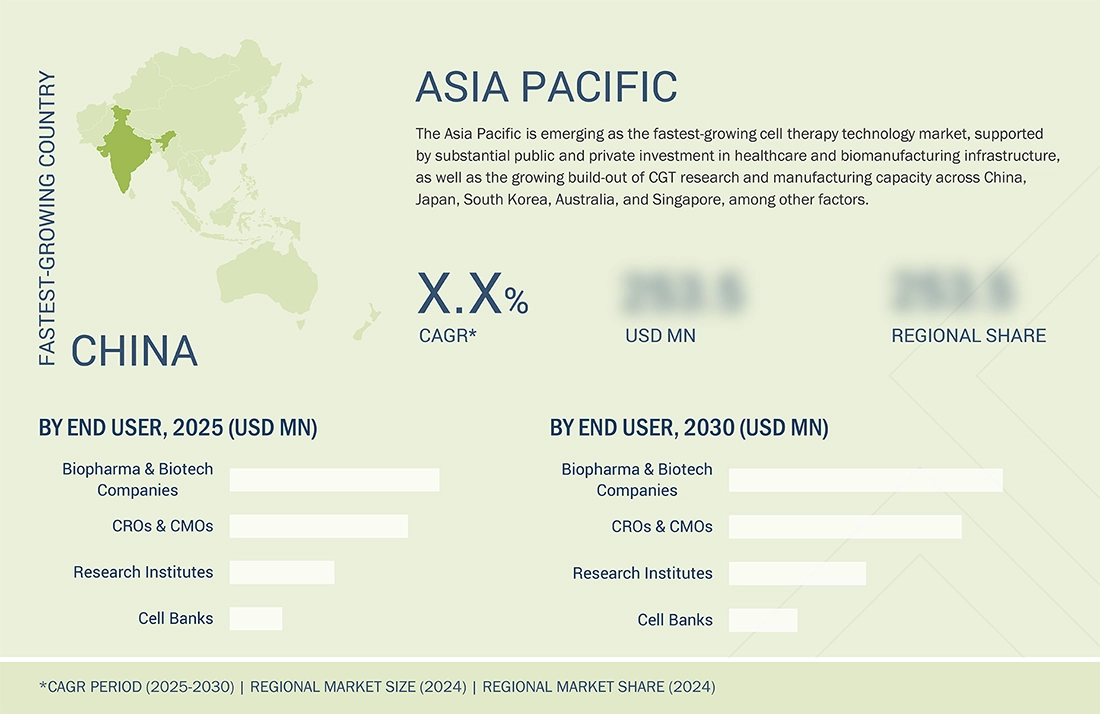
ASIA PACIFIC: CELL THERAPY TECHNOLOGIES MARKET SNAPSHOT
FIGURE 34
REVENUE ANALYSIS OF KEY PLAYERS IN CELL THERAPY TECHNOLOGIES MARKET, 2021–2023
FIGURE 35
MARKET SHARE ANALYSIS OF KEY PLAYERS IN CELL THERAPY TECHNOLOGIES MARKET, 2023
FIGURE 36
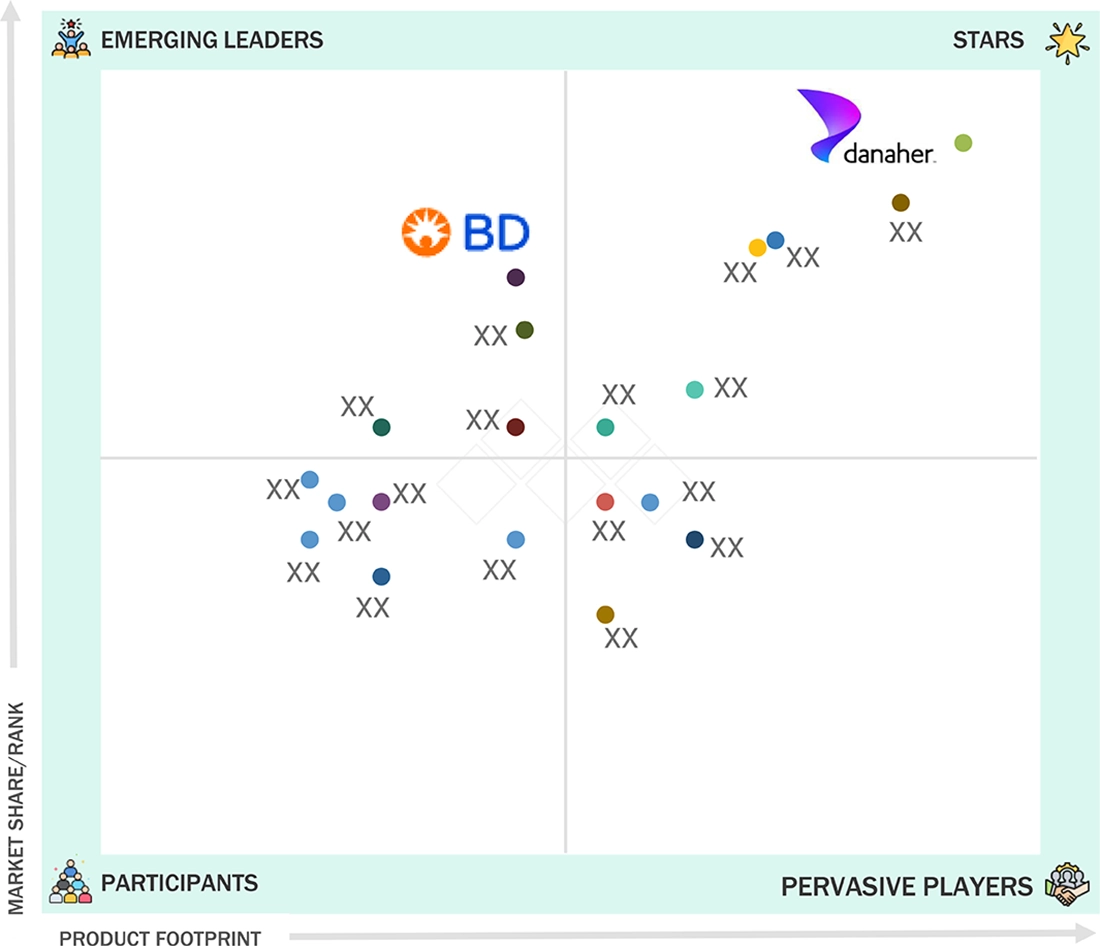
CELL THERAPY TECHNOLOGIES MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
FIGURE 37
CELL THERAPY TECHNOLOGIES MARKET: COMPANY FOOTPRINT
FIGURE 38
CELL THERAPY TECHNOLOGIES MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2023
FIGURE 39
EV/EBITDA OF KEY VENDORS
FIGURE 40
YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
FIGURE 41
CELL THERAPY TECHNOLOGIES MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
FIGURE 42
DANAHER CORPORATION: COMPANY SNAPSHOT
FIGURE 43
MERCK KGAA: COMPANY SNAPSHOT
FIGURE 44
THERMO FISHER SCIENTIFIC INC: COMPANY SNAPSHOT
FIGURE 45
LONZA: COMPANY SNAPSHOT
FIGURE 46
SARTORIUS AG: COMPANY SNAPSHOT
FIGURE 47
AGILENT TECHNOLOGIES: COMPANY SNAPSHOT
FIGURE 48
AVANTOR: COMPANY SNAPSHOT
FIGURE 49
FRESENIUS: COMPANY SNAPSHOT
FIGURE 50
BD: COMPANY SNAPSHOT
FIGURE 51
CORNING INCORPORATED: COMPANY SNAPSHOT
FIGURE 52
TERUMO CORPORATION: COMPANY SNAPSHOT
FIGURE 53
BIO-TECHNE: COMPANY SNAPSHOT
FIGURE 54
GENSCRIPT: COMPANY SNAPSHOT
FIGURE 55
MAXCYTE: COMPANY SNAPSHOT





















Growth opportunities and latent adjacency in Cell Therapy Technologies Market