
Elastomeric Infusion Pumps Market Size, Growth, Share & Trends Analysis
Elastomeric Infusion Pumps Market by Type (Continuous Rate and Variable Rate), Application (Chemotherapy, Pain Management, Antibiotics), End-use Industry (Hospitals, Ambulatory Surgical Centers, Home-care Settings), and Region - Global Forecast to 2030
Updated on : November 27, 2025




ELASTOMERIC INFUSION PUMPS MARKET OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
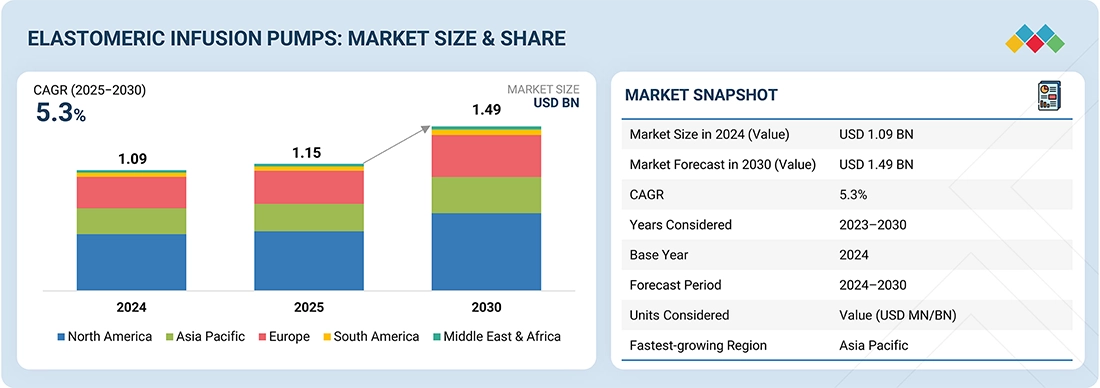
The global elastomeric infusion pumps market, valued at US$1.09 billion in 2024, stood at US$1.15 billion in 2025 and is projected to advance at a resilient CAGR of 5.3% from 2025 to 2030, culminating in a forecasted valuation of US$1.49 billion by the end of the period. The growing prevalence of chronic illness, aging population, and rising surgical procedures drive the need for reliable, patient-friendly drug delivery systems. Elastomeric infusion pumps offer simplicity, portability, and cost-effectiveness for continuous or variable-rate medication, suitable for ambulatory and home care. Advances in biocompatible materials, smart sensors, customizable flow rates, and green manufacturing will enable more precise delivery, reduce healthcare costs, and improve outcomes for continuous therapy. Cancer remains a main driver, with around 9.7 million deaths globally in 2022. The CDC projects 2,001,140 new cancers and about 611,720 deaths in 2024. Projections show 29.9 million new cancer cases and 15.3 million deaths by 2040 (Source: WHO). Elastomeric infusion pumps are vital for chemotherapy, pain management, and long-term therapy, supporting the shift toward more effective, patient-centered healthcare.
KEY TAKEAWAYS
-
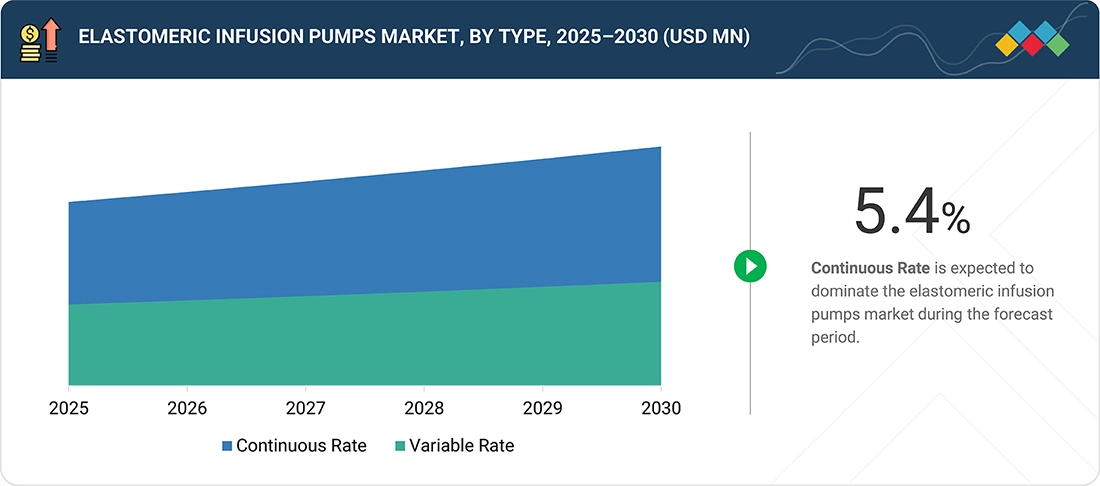
BY TYPEThe elastomeric infusion pump market is segmented into continuous-rate and variable-rate types. Continuous rate pumps lead the market by providing consistent, predetermined medication flow, ideal for pain management and long-term therapy.
-
BY APPLICATIONThe elastomeric infusion pumps market is segmented by application into pain management, chemotherapy, antibiotics, and other applications. Antibiotics represent the fastest-growing segment, driven by rising outpatient treatments and home-based infection management.
-
BY END-USE INDUSTRYThe elastomeric infusion pumps market is segmented by end-use into Hospitals, Ambulatory Surgical Centers (ASCs), Home-Care Settings, and Other industries. Hospitals hold the largest share, while Home-Care Settings are the fastest-growing segment due to demand for convenient, patient-friendly drug delivery.
-
BY REGIONThe elastomeric infusion pumps market is segmented regionally into North America, Europe, Asia Pacific, South America, and the Middle East & Africa. Asia Pacific is the fastest-growing region, driven by rising chronic disease prevalence, an aging population, increasing surgical procedures, and growing awareness of ambulatory and home healthcare solutions.
-
COMPETITIVE LANDSCAPEThe major market players have adopted both organic and inorganic strategies, including acquisitions, partnerships, and expansions. For instance, Baxter, B. Braun SE, NIPRO, AVNS, and Vygon entered into several agreements, partnerships, and expansions to cater to the growing demand for elastomeric infusion pumps across various innovative applications.
Factors contributing to the growth of the elastomeric infusion pumps market include an increase in the prevalence of chronic diseases and demand for outpatient and home-based care. The increasing prevalence of surgical interventions and chemotherapy necessitates the development of accurate, continuous, and patient-centric drug administration systems. Furthermore, innovations in pump design, including mobility, mechanical simplicity, and adjustable flow rates, are facilitating acceptance in hospitals, ambulatory surgery centers, and home healthcare environments. Other market drivers include increasing patient awareness, rising reimbursement, and the shift toward cost-effective and patient-centered care.
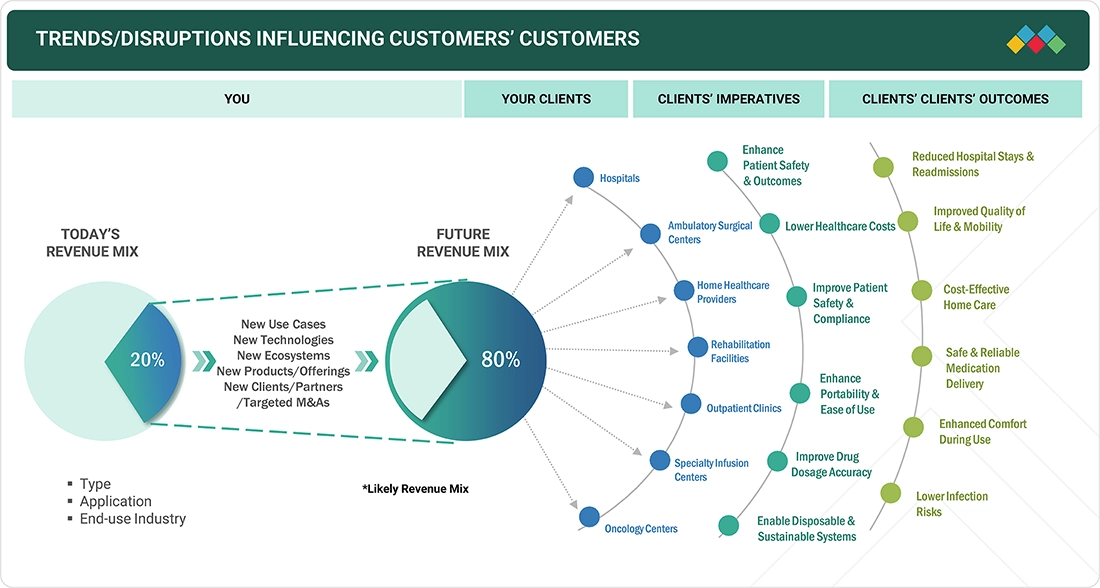
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The influence on healthcare providers and patients in the elastomeric infusion pumps market is driven by increasing demand for outpatient care, rising prevalence of chronic and acute conditions requiring continuous drug delivery, and the growing adoption of home healthcare solutions. Key end-use segments include hospitals, ambulatory care centers, oncology clinics, and home healthcare settings. Targeted applications focus on pain management, chemotherapy, antibiotic therapy, and post-surgical care. Fluctuations in end-user demand directly affect revenues for manufacturers and suppliers of elastomeric infusion pumps, thereby shaping overall market growth and sales dynamics.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
ELASTOMERIC INFUSION PUMPS MARKET DYNAMICS
Level
-
Rising prevalence of chronic diseases

-
Growing shift to ambulatory and home-based care
Level
-
Rising risks of incorrect drug delivery and performance variability
-
Regulatory recalls on elastomeric pump reliability
Level
-
Rising healthcare advancements in emerging economies
-
Specialization for high-viscosity drugs and biologics
Level
-
Intense competition with electronic infusion technologies
-
Maintaining precision and safety in scaled elastomeric pump production
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising Prevalence of Chronic Diseases
Rising chronic diseases drive the elastomeric infusion pump market as these conditions need reliable, ambulatory drug delivery, reducing hospital dependence. Such diseases—cardiovascular, neurological, cancer, diabetes, respiratory, renal—require precise, continuous medication. These small, battery-free devices deliver therapies like chemotherapy, insulin, antibiotics, and pain relief, improving mobility and reducing healthcare costs. They support cancer, thalassemia, and infection treatments. In 2024, the National Cancer Institute reported 2 million new cancer cases and over 600,000 deaths; the CDC states 45% of US adults have chronic diseases. Globally, 75% of deaths are from chronic conditions. This trend drives demand for safe and effective drug delivery systems, such as elastomeric pumps.
Restraint: Rising risks of incorrect drug delivery and performance variability
Elastomeric infusion pumps are vital for delivering biologics and high-viscosity drugs in outpatient settings. However, their effectiveness is compromised by inaccuracies and safety issues, as factors like environment temperature, drug viscosity, and user setup errors can alter flow rates. Data from the US FDA shows flow rates can rise about 2.3% per 1°C increase, risking over-infusion. The NIH reports viscosity impacts flow in nearly 72% of biologics. Material incompatibility and human errors, causing 5–62.9% of IV errors and 237 million drug errors annually per WHO, further limit use. Issues like cold under-infusion, cannula blockages, and flow variability are documented. Solutions include improved elastomer chemistries, better material compatibility, mechanized testing, and electronic health record integration, especially for chronic and oncology care.
Opportunity: Rising healthcare advancements in emerging economies
India, China, and Brazil are among the emerging economies that offer significant growth potential for elastomeric infusion pumps, driven by the growing prevalence of chronic diseases, cancer, and rising demand for home- and hospital-based care. India alone has 77 million type-2 diabetes adults, and there are more than 140 million in China, which explains the need for controlled and continuous drug administration (Sources: WHO, World Diabetes Foundation). Cancer diagnoses are also increasing steeply, with India recording 1.4 million new cases in 2023 and China 4.8 million in 2022. The number of cancer cases is also expected to rise significantly to 6.85 million by 2040 (Sources: ICMR, IARC). Neonatal and maternal health requirements impose additional pressure, as low-birth-weight and preterm babies need accurate, uninterrupted infusion. In addition, public and private sector government programs, such as India’s national diabetes program and China’s Healthy China 2030 policy, combined with low-cost manufacturing and increased healthcare infrastructure, support the growth and adoption of elastomeric infusion pumps. Their portability, ease of use, and entirely maintenance-free operation make elastomeric infusion pumps best suited for outpatient, home-based care, and decentralized medical care associated with such emerging high-need regions.
Challenge: Intense competition with electronic infusion technologies
Elastomeric infusion pumps are valued for their independence and portability, but they face competition from electronic options that offer higher precision and versatility. Electronic pumps enable programmable flow rates (0.1–125 mL/hour) and alarms for occlusion or malfunction, allowing for real-time adjustments, unlike fixed-rate elastomeric devices that lack alerts. NIH research indicates an elastomeric accuracy of +10–15%, but actual variations can reach +50% due to temperature, height, and back pressure, potentially leading to under- or overdosing of biologics and antibiotics. Residual volumes after infusion limit dose delivery. Laboratory tests reveal elastomeric flow deviations of up to 2.4 times during height changes of +40 cm, while electronic pumps remain unaffected. The CHID study found that 35–47% of elastomeric infusions had over a 15% variation due to temperature changes. These limitations highlight the need for improved elastomeric materials to support more reliable, precise, and adaptable devices for oncology and chronic care, as recommended by ISPE.
Elastomeric Infusion Pumps Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
A 46-year-old patient received weekly 5-hour magnesium and calcium infusions via a balloon pump instead of a traditional electronic pump and drip stand. The pump was easy to set up, compact, and allowed mobility during treatment. | Improved patient mobility and comfort, increased satisfaction, easier setup after practice, and reduced hindrance compared to traditional infusion methods. |
 |
Elastomeric pumps were used for postoperative patient-controlled analgesia (PCA) as a cost-effective, mobile alternative to electric PCA pumps. The pumps provide continuous or adjustable basal/bolus analgesia without electricity, supporting systemic or regional pain management in hospital and outpatient settings. | Significant cost savings (up to 50–70% per patient in low-volume settings), enhanced patient mobility and satisfaction, reduced personnel requirements, multi-day use without extra disposables, lower infection risk, and improved economic efficiency compared to electric pumps. |
 |
A 51-year-old colorectal cancer patient used the Surefuser+ ambulatory pump for a 46-hour Fluorouracil infusion via a PICC line at home. The pump allowed discreet, uninterrupted infusion, with minimal nurse intervention and patient education for home use. | Eliminated a 2-day hospital stay, improved sleep quality, supported daily activities, enhanced patient comfort and satisfaction, and maintained normal routines during chemotherapy. |
 |
Offers EZ-FLOW™ elastomeric pumps for chemotherapy and long-duration infusion therapies. | Eliminates need for pinch clamps; simplifies priming process; ensures consistent drug delivery. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
ELASTOMERIC INFUSION PUMPS MARKET ECOSYSTEM
An ecosystem map for the elastomeric infusion pumps market highlights the interconnected network of stakeholders across its value chain. It includes raw material suppliers (such as medical-grade elastomers, plastics, flow restrictors, and connectors), elastomeric infusion pump manufacturers, distributors, and end-use healthcare providers, including hospitals, ambulatory surgical centers, home-care services, oncology clinics, and specialty care facilities.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
ELASTOMERIC INFUSION PUMPS MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Elastomeric Infusion Pumps Market, By Type
In 2024, the continuous rate segment led the elastomeric infusion pumps market, led by its reliability and consistent medication administration. These pumps are widely used in hospitals, outpatient care centers, and home healthcare settings, offering accurate and consistent medicine delivery without the need for electronic programming. Continuous-rate elastomeric pumps are particularly advantageous for pain control, chemotherapy, and antibiotic administration, where it is critical to maintain a constant infusion rate for the efficacy of treatment and the safety of patients. Their ease of use, convenience, and low maintenance encourage their use, particularly in areas with limited healthcare infrastructure.
Elastomeric Infusion Pumps Market, By Application
In 2024, pain management dominated the elastomeric infusion pumps market. Common uses include postoperative, chronic, and palliative pain relief, providing controlled analgesic delivery. Their portability and ease support outpatient and home care, reducing hospital visits and increasing comfort. Growing surgical procedures, an aging population, and better pain management knowledge have driven demand. New pump designs with improved control and user features have advanced their use in acute care, making pain management the largest and fastest-growing application globally.
Elastomeric Infusion Pumps Market, By End-use Industry
In 2024, hospitals dominated the elastomeric infusion pumps market, holding the largest share among end-use sectors. They are essential for reliable, continuous, and controlled drug delivery in post-operative care, pain management, chemotherapy, and antimicrobial therapy. Hospitals favor elastomeric pumps for their simplicity, portability, and low maintenance, supporting patient-centered care with less dependence on computerized infusion modes. The rising number of surgeries, expanding patient population, and focus on better outcomes have boosted their use. Leading healthcare providers heavily rely on these pumps, maintaining hospitals as the primary end-use in the global market.
REGION
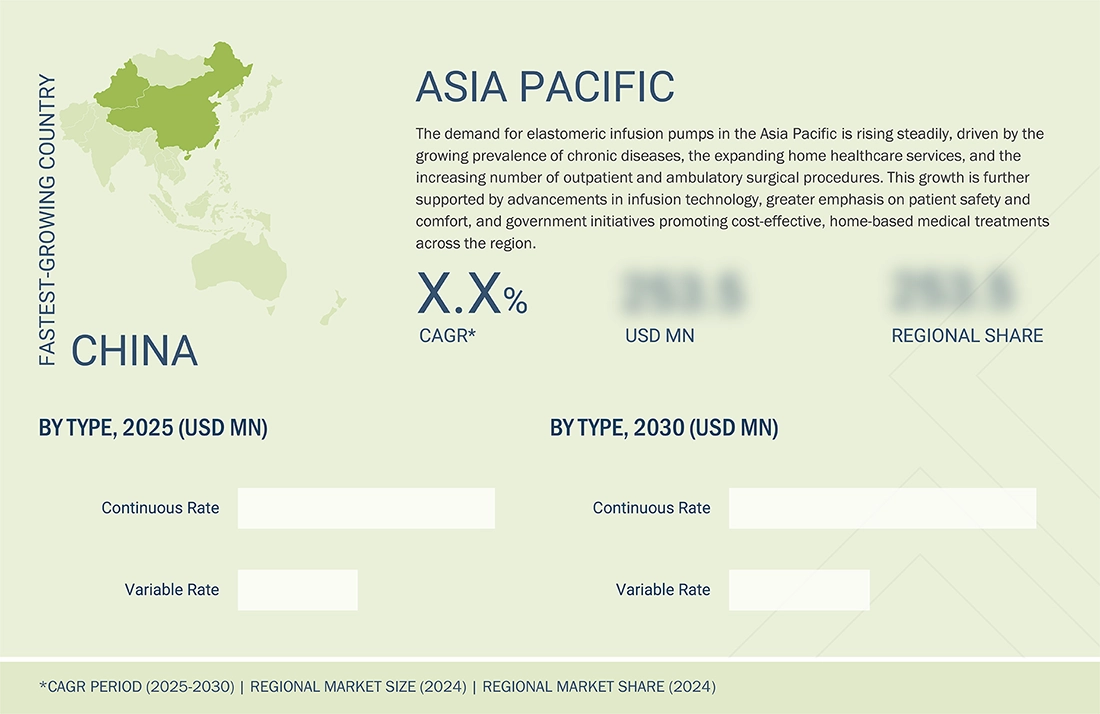
Asia Pacific to be the fastest-growing region in the global elastomeric infusion pumps market during the forecast period
The Asia Pacific region is projected to be the fastest-growing market for elastomeric infusion pumps, driven by increased demand for portable, patient-centric drug delivery systems. One in four people in Asia and the Pacific will reach 60 years of age or older by 2050, according to UNFPA projections, which show that the number of older adults will increase threefold from 2010 to 2050, exceeding 1.3 billion people. This demographic transition is also leading to an increased incidence of chronic conditions like cancer, diabetes, and cardiovascular diseases that need consistent and accurate drug delivery. The increase in healthcare infrastructure, government access initiatives, and growing interest in infusion therapies are driving the use of elastomeric pumps in home healthcare and outpatient settings.
Elastomeric Infusion Pumps Market: COMPANY EVALUATION MATRIX
Baxter (Star) holds a strong position in the elastomeric infusion pumps market, with a broad geographical presence, extensive product portfolio, and investment in research and development, leading to widespread adoption in hospitals, ambulatory care centers, oncology clinics, and home healthcare settings. WOOYOUNG MEDICAL (Emerging Leader) has begun capturing a portion of the market by offering innovative, high-performance, and patient-friendly infusion solutions, all while designing pumps with customizability for pain management, chemotherapy, and antibiotic therapy.
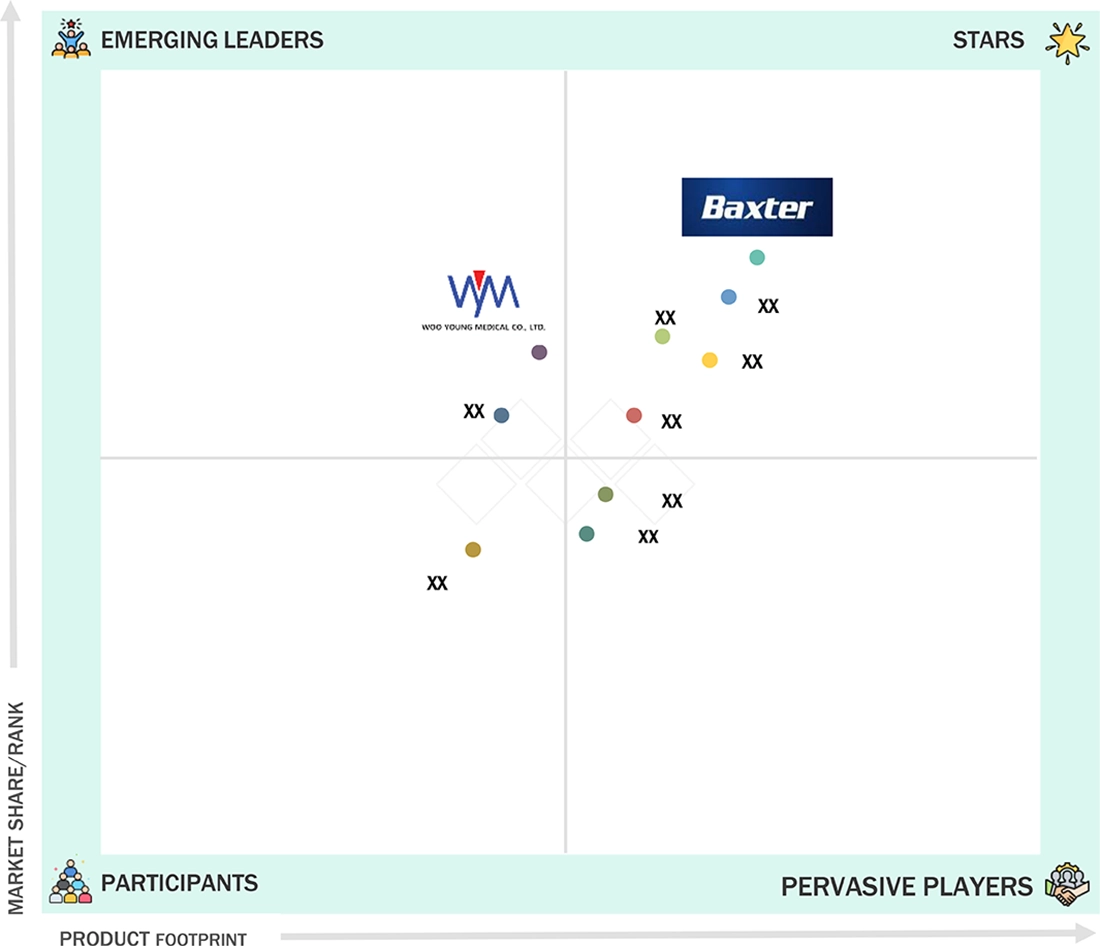
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2023 (Value) | USD 1.09 Billion |
| Market Forecast in 2029 (Value) | USD 1.49 Billion |
| CAGR | 5.3% |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD MN/BN) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | North America, Asia Pacific, Europe, South America, Middle East & Africa |
WHAT IS IN IT FOR YOU: Elastomeric Infusion Pumps Market REPORT CONTENT GUIDE
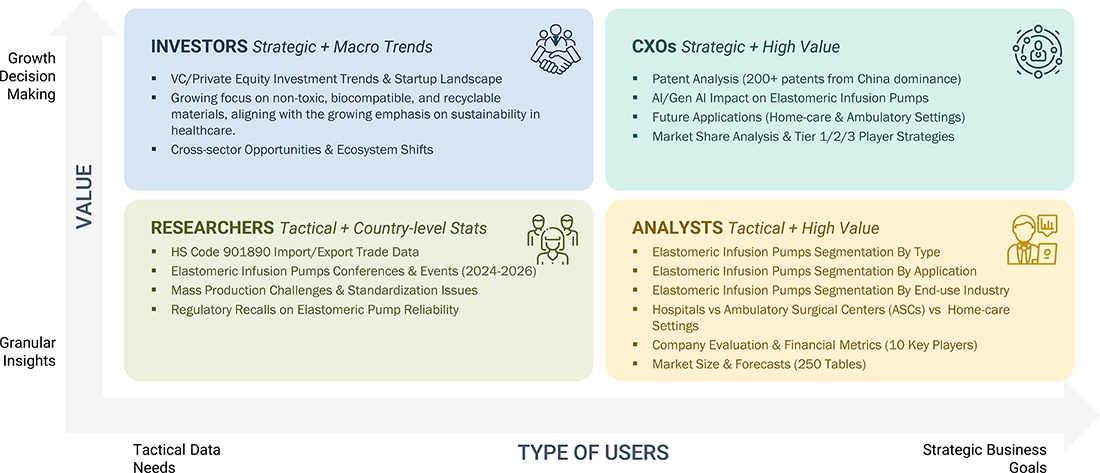
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| US-based elastomeric infusion pumps manufacturer |
|
|
| Continuous rate elastomeric infusion pumps manufacturers |
|
|
| Variable rate elastomeric infusion pumps manufacturers |
|
|
RECENT DEVELOPMENTS
- July 2025 : Vygon partnered with Ecovamed, a company specializing in assessing and reducing the environmental impact of medical devices. This collaboration enables Vygon to calculate a “Green Score” for its products, enhancing transparency, supporting eco-labeling initiatives, and advancing sustainable healthcare practices.
- October 2024 : Nipro Trading (Shanghai), a subsidiary of NIPRO, acquired a 70% stake in Sichuan Pure Science and Technology Co., Ltd., a manufacturer of dialysis and infusion-related equipment.
- July 2024 : Nipro Medical Corporation, a subsidiary of NIPRO, has secured a site in Pitt County, North Carolina, for a new medical device manufacturing plant.
- October 2022 : NIPRO established Nipro Medical Philippines Corp., with operations scheduled to begin in January 2023. The subsidiary will directly sell Nipro medical devices
- March 2022 : B. Braun acquired Intermedt Medizin & Technik GmbH, a specialist in dialysis concentrate preparation systems. This acquisition strengthens B. Braun’s expertise in dialysis solutions and complements its infusion therapy and elastomeric pump portfolio by enhancing its integrated care offerings for renal and infusion treatments.
Table of Contents

Methodology
The study involved four major activities to estimate the current size of the global elastomeric infusion pumps market. Exhaustive secondary research was conducted to gather information on the market, the peer product market, and the parent product group market. The next step was to validate these findings, assumptions, and sizes with the industry experts across the value chain of elastomeric infusion pumps through primary research. The top-down and bottom-up approaches were employed to estimate the overall size of the elastomeric infusion pumps market. After that, market breakdown and data triangulation procedures were used to determine the size of different segments and sub-segments of the market.
Secondary Research
The market for companies offering elastomeric infusion pumps is determined by secondary data obtained from paid and unpaid sources, analyzing the product portfolios of major companies in the ecosystem, and evaluating companies based on their performance and quality. Various secondary sources, including Business Standard, Bloomberg, the World Bank, and Factiva, were consulted to identify and collect information for this study on the elastomeric infusion pumps market. During the secondary research process, various secondary sources were consulted to identify and collect information relevant to the study. Secondary sources included annual reports, press releases, investor presentations of door vendors, forums, certified publications, and white papers. The secondary research was utilized to gather critical information on the industry’s value chain, the total pool of key players, market classification, and segmentation from both market- and technology-oriented perspectives.
Primary Research
In the primary research process, various primary sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side included industry experts, such as Chief Executive Officers (CEOs), Vice Presidents (VPs), marketing directors, technology and innovation directors, and related key executives from several key companies and organizations operating in the elastomeric infusion pump market. After completing the market engineering process (calculations for market statistics, market breakdown, market size estimations, market forecasting, and data triangulation), extensive primary research was conducted to gather information and verify and validate the critical numbers derived. Primary research was also conducted to identify the segmentation types, industry trends, competitive landscape of elastomeric infusion pumps offered by various market players, and key market dynamics, such as drivers, restraints, opportunities, challenges, industry trends, and key player strategies. In the complete market engineering process, the top-down and bottom-up approaches and several data triangulation methods were extensively used to perform the market estimation and market forecasting for the overall market segments and subsegments listed in this report. Extensive qualitative and quantitative analysis was performed on the complete market engineering process to list the key information/insights throughout the report.
The following is the breakdown of primary respondents:
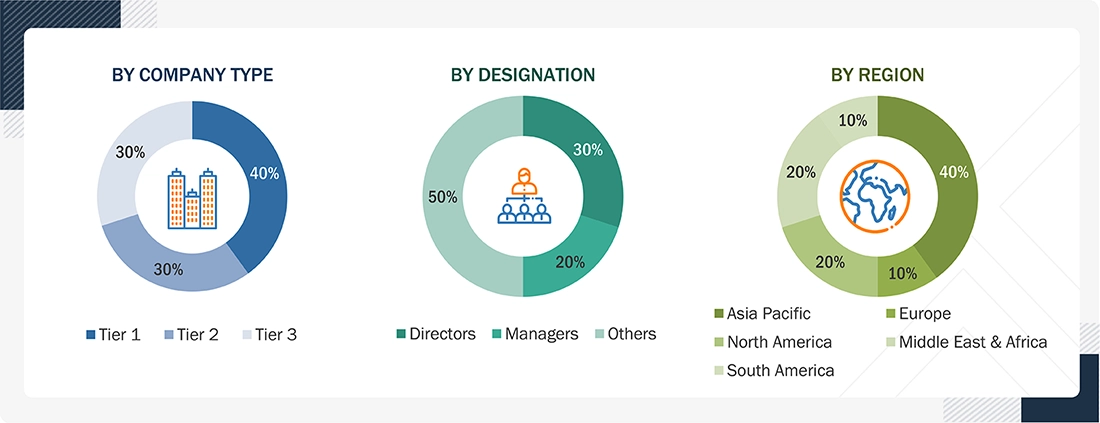
Notes: Other designations include sales, marketing, and product managers. Tier 1: >USD 1 Billion; Tier 2: USD 500 million–1 Billion; and Tier 3: < USD 500 million.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The top-down and bottom-up approaches were employed to estimate and validate the size of the global elastomeric infusion pumps market. These methods were also widely used to assess the size of various related market segments. The research methodology used to determine the market size included the following:
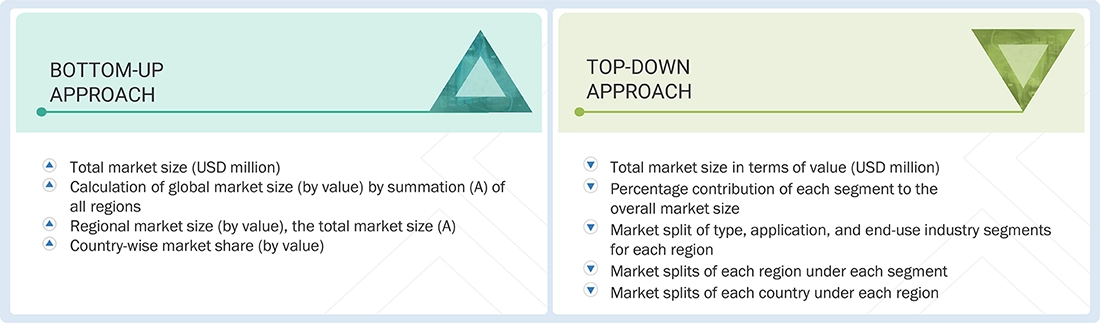
Data Triangulation
After determining the overall market size using market size estimation processes, the market was segmented into several segments and subsegments. The data triangulation and market breakup procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Elastomeric infusion pumps are portable, non-electronic medical devices designed for the controlled delivery of drugs, such as analgesics, antibiotics, and chemotherapeutic agents, over a specified period. These devices utilize the elastic properties of specific elastomers to deliver accurate, continuous, or intermittent medication infusions without requiring batteries or external power sources. Elastomeric infusion pumps are recognized for their simplicity, reliability, and ease of use, making them suitable for use in hospital, outpatient, and home care environments. They are produced and tested in accordance with international medical device standards, including ISO 13485 for quality management, ISO 11608 for infusion device efficacy, and ASTM standards for elastomeric materials. The devices are extensively utilized in oncology, pain management, anesthesia, and palliative care, enhancing patient compliance, mobility, and safety. Elastomeric infusion pumps are crucial to the advancement of the field of patient-centered drug delivery and healthcare innovation, particularly in home healthcare and ambulatory care.
Stakeholders
- Elastomeric infusion pump manufacturers
- Raw material suppliers
- Converters & processors
- Distributors and traders
- Industry associations and regulatory bodies
- End users
Report Objectives
- To define, describe, and forecast the size of the global elastomeric infusion pumps market, based on type, end-use industry, and region in terms of value and volume
- To provide detailed information on the significant drivers, restraints, opportunities, and challenges influencing the market
- To strategically analyze micromarkets concerning individual growth trends, prospects, and their contribution to the market
- To assess the growth opportunities in the market for stakeholders and provide details on the competitive landscape for market leaders
- To forecast the market size of segments and subsegments for North America, Europe, Asia Pacific, South America, and the Middle East & Africa
- To strategically profile key players and comprehensively analyze their market shares and core competencies
- To analyze competitive developments such as acquisitions, expansions, partnerships, and agreements in the elastomeric infusion pumps market
- To provide the impact of AI/Gen AI on the market
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Elastomeric Infusion Pumps Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Elastomeric Infusion Pumps Market