
Europe Dental Bone Graft Substitutes Market Size, Growth, Share & Trends Analysis
Europe Dental Bone Graft Substitutes Market by Type (Xenograft, Allograft, Synthetic Bone Grafts, Alloplast), Application (Sinus Lift, Ridge Augmentation, Socket Preservation), Product (BioOss, Osteograf, Grafton), End User, Country - Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
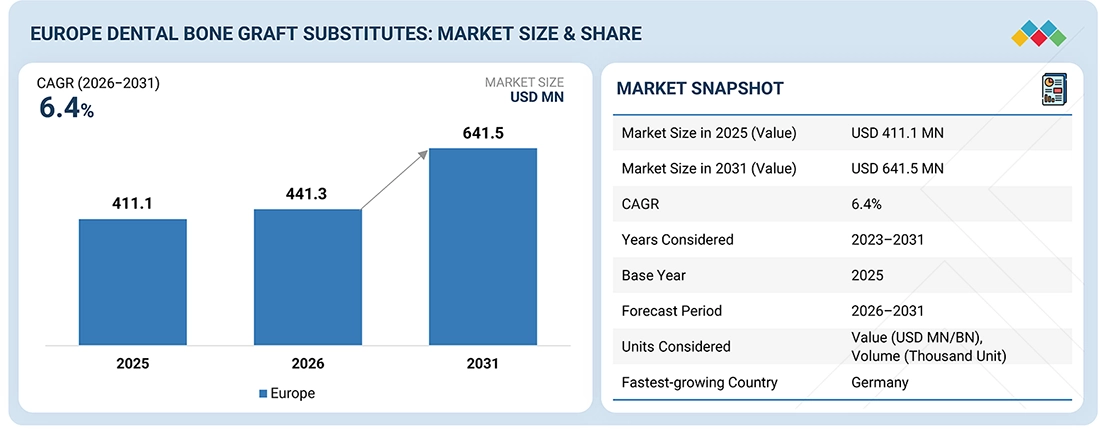
The Europe dental bone graft substitutes market is expected to reach USD 641.5 million by 2031, up from USD 411.1 million in 2025, at a CAGR of 6.4% during the forecast period. The main factors driving this rise are the increased use of advanced regenerative materials in implantology and periodontal procedures. Dental bone graft substitutes are the materials, both biologic and synthetic, that aim at restoration, augmentation, or preservation of alveolar bone volume in such applications as socket preservation, ridge augmentation, sinus lift, and implant site development. Their sources may be xenografts, allografts, synthetic grafts, or composite materials; whichever is used, the materials are designed to support osteoconduction and predictable bone regeneration. Various dental hospitals, clinics, and specialized laboratories across Europe are extensively using bone graft substitutes in routine and complex implant workflows to achieve better clinical outcomes, enhance implant stability, and shorten treatment time; thus, secondary grafting procedures are less necessary. The trend towards minimally invasive treatments, standardized regenerative outcomes, and shortened treatment timelines has therefore elevated the adoption of dental bone graft substances across the region.
KEY TAKEAWAYS
-
BY COUNTRYBy country, Germany garnered the highest share in the Europe dental bone graft substitutes market.
-
BY PRODUCTBy product, the BioOss segment is projected to increase at the highest CAGR during the forecast period in the Europe dental bone graft substitutes market.
-
BY TYPEBy type, the synthetic bone graft substitutes segment is projected to increase at the highest CAGR during the forecast period in the Europe dental bone graft substitutes market.
-
BY APPLICATIONBy application, the periodontal defect regeneration segment is projected to increase at the highest CAGR in the Europe dental bone graft substitutes market.
-
By END USERBy end user, the hospitals segment garnered the highest share in the Europe dental bone graft substitutes market.
-
COMPETITIVE LANDSCAPE- KEY PLAYERSDentsply Sirona, Envista, and Medtronic were identified as some of the star players in the Europe dental bone graft substitutes market, given their strong market share and product/service footprint.
-
COMPETITIVE LANDSCAPE- STARTUPSCompanies such as Kervalion, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The dental bone graft substitutes market in Europe is driven by the rising prevalence of tooth loss, periodontal diseases, and age-related bone resorption. The increasing demand for predictable implant outcomes and esthetic restorations is driving the adoption of bone graft substitutes in implant and regenerative procedures. Additionally, the growing use of digital treatment planning and guided workflows in dental clinics is improving procedural accuracy and accelerating the shift toward advanced bone regeneration solutions across Europe.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Europe dental bone graft substitutes market is witnessing significant changes, mainly driven by the increased use of advanced regenerative techniques, expanded clinical applications, and the ongoing innovation in the regional dental ecosystem. The transition of clinical practices in dental hospitals, clinics, and implant centers across Europe is being influenced by the abandonment of conventional grafting approaches and the use of ready-to-use synthetic, xenograft, and bioengineered substitutes. More predictable bone regeneration, less surgical complexity, and improved implant success rates are the results of innovations in biomaterial design, osteoconductive properties, and handling characteristics. At the same time, the upgradation of treatment planning through digital integration, guided surgery, and standardization of clinical protocols is improving procedural efficiency and outcome consistency. These changes, in fact, are leading to shorter treatment times, better-quality regenerative results, and improved patient satisfaction which, in turn, are driving the market’s expansion across the region.

Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising cases of dental caries and the subsequent increase in tooth repair procedures

-
Growing market for dental tourism in many countries
Level
-
Lack of proper reimbursement scenario
-
Stringent regulations pertaining to medical devices used in dentistry
Level
-
Consolidation of dental practices and rising DSO activity
-
Increasing demand from customers and rising inclination toward cosmetic dentistry
Level
-
Pricing pressure faced by prominent market players
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising cases of dental caries and subsequent increase in tooth repair procedures
The Europe market of dental bone graft substitutes is mainly influenced by the high and increasing rate of dental caries and other oral diseases that, in most cases, lead to tooth extractions and complex restorative treatments. According to the WHO European Region, as early as 2019, more than half of the adult population was severely affected by oral disease; thus, it had the highest prevalence in the world. The large disease burden, coupled with limited preventive frameworks, as indicated by the lack of national oral health policies in most European countries and the relatively low per-capita spending on dental care, has led to an increase in tooth repair, extraction, and implant procedures. Since bone loss is the most common side effect of tooth extraction, the rising number of restorative and implant-related interventions is the main reason for the increasing demand for dental bone graft substitutes that are used to preserve alveolar bone, support implant placement, and give a better long-term clinical outcome.
Restraint: Lack of proper reimbursement scenario
The rise of the Europe dental bone graft substitutes market is constrained by the region's varying reimbursement systems for dental procedures. In many European countries, implant-related bone grafting, periodontal regeneration, or ridge augmentation is only partially reimbursed or even completely excluded from public insurance coverage, so patients have to pay a significant portion out of their own pockets. This extra cost is a reason advanced bone graft substitute materials are not adopted, especially among patient groups sensitive to price. Moreover, fragmented, country-specific reimbursement policies lead to differences in the level of treatment and thus limit dental clinics' investments in high-end grafting solutions, which, in turn, slow the spread of dental bone graft substitutes in Europe.
Opportunity: Consolidation of dental practices and rising DSO activity
The market for dental bone graft substitutes will greatly benefit from the consolidation trend of dental practices and the growth of Dental Service Organizations (DSOs) in Europe. Although the DSO environment in Europe remains fragmented and regulated more strictly than in the US, dental groups of significant size in the UK, Germany, Spain, and the Nordic countries, for instance, are increasingly widening their reach by way of takeovers. Through this merger, clinics have the opportunity to achieve economies of scale, standardize clinical protocols, and invest in advanced treatment capabilities, for example, implantology and regenerative procedures that involve the use of bone graft substitutes. Group practices and DSOs, which are supported by better capital access and centralized procurement models, have a greater potential to take up top-notch grafting materials and be in a position to carry out a higher number of procedures. As consolidation gets into gear, it will likely be the main driver of increased use of dental bone graft substitutes, driven by standard care pathways, more implant placements, and better operational efficiency in European dental networks.
Challenge: Pricing pressure faced by prominent market players
Leading companies in the Europe dental bone graft substitutes market are experiencing a considerable price competition as their competitors become more numerous and customers become more cost-conscious. Large dental groups and DSOs have changed the purchasing power structure, giving end users greater power. Centralized procurement teams negotiate prices aggressively and, at the same time, request volume discounts. Meanwhile, a wider range of alternative graft products, such as xenografts and synthetic substitutes, gives clinics the freedom to switch suppliers; thus, margins are declining. Moreover, these difficulties are intensified by high regulatory and compliance costs under the EU MDR, which significantly limit manufacturers’ ability to raise prices. As a result, market players are challenged to maintain profitability while continuing to invest in innovation, quality assurance, and clinical evidence.
EUROPE DENTAL BONE GRAFT SUBSTITUTES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Symbios Xenograft Granules are used for bone regeneration in implant dentistry, including socket preservation, ridge augmentation, and sinus lift procedures. | High osteoconductivity | Natural bone-like structure | Predictable bone regeneration | Strong clinical acceptance in implant workflows |
 |
creos xenoprotect is a collagen-based xenograft membrane used in guided bone regeneration (GBR) and guided tissue regeneration (GTR) procedures. | Effective barrier function | Controlled resorption | Improved soft-tissue healing | Enhanced graft stability |
 |
ChronOS Bone Graft Substitute is a synthetic calcium phosphate-based graft used for bone void filling and regenerative dental applications. | Consistent quality | Biocompatibility | Eliminates donor-related risks | Predictable resorption | Bone remodeling |
 |
Straumann xenograft bone substitutes are used for implant site development, socket preservation, and sinus augmentation procedures. | Excellent volume stability | Long-term scaffold support | High implant success rates | Strong integration with Straumann implant system |
 |
IngeniOs HA Bone Graft Substitute is a synthetic hydroxyapatite-based graft used in dental implant and periodontal bone regeneration. | High structural stability | Slow resorption for volume maintenance | Reliable handling characteristics | Suitable for load-bearing sites |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe dental bone graft substitutes market is a complex network of stakeholders, including biomaterial suppliers, manufacturers, regulators, distributors, and end users. A variety of implant and periodontal procedures, combined with synthetic, xenograft, and composite graft materials, enhance bone regeneration, led by companies such as Straumann Group, Dentsply Sirona, Envista, Johnson & Johnson, and ZimVie. These materials are offered to dentists through established dental distributors and specialist suppliers who have strong regional logistics networks. Market access and commercialization are regulated by the EU Medical Device Regulation (MDR 2017/745) and local authorities, whereas country-specific reimbursement schemes determine purchasing and adoption decisions. End users primarily include hospitals, dental clinics, research institutes, and academic centers, with clinical adoption facilitated by key opinion leaders in implantology and regenerative dentistry.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
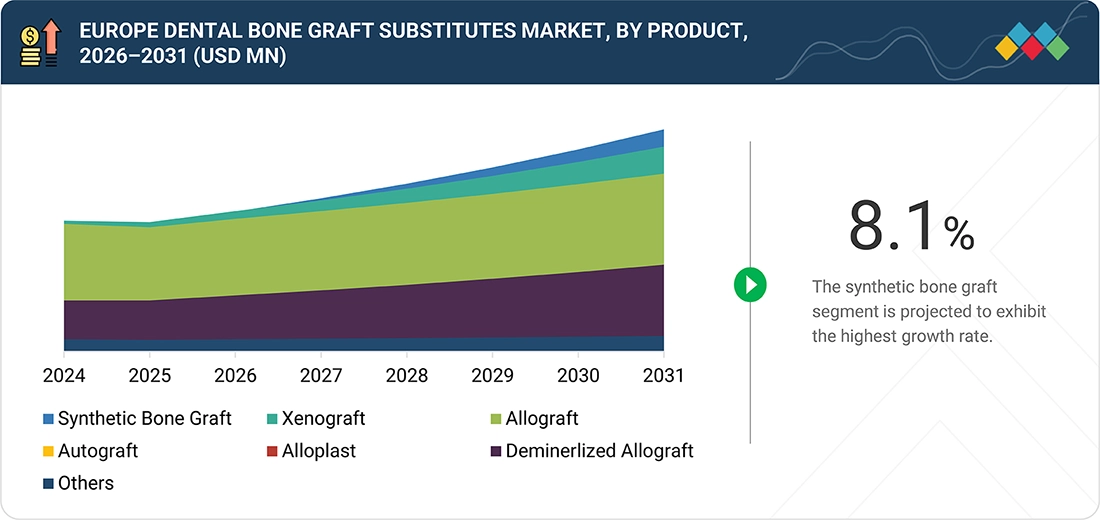
Europe Dental Bone Graft Substitutes Market, By Type
The type segment is segmented into synthetic bone grafts, xenografts, allografts, autografts, alloplasts, demineralized allografts, and others. Synthetic bone grafts constitute a crucial and rapidly expanding segment of the dental bone graft substitutes market, offering a convenient, standardized, and safe alternative to biological grafts. The removal of donor-related limitations through their off-the-shelf availability makes them the most suitable dental and implant procedures for disease transmission, thereby eliminating the risk. In fact, the present and future clinical results are improving due to ongoing advancements in biomaterials—such as improved osteoconductivity, controlled resorption rates, and enhanced handling properties. By the time manufacturing technologies become fully developed and prices gradually decrease, synthetic bone grafts will have a significant role in jawbone reconstruction and implant placement procedures, thus, segment growth will be accelerated.
Europe Dental Bone Graft Substitutes Market, By Application
The usability segment is segmented into fixed and removable dentures. Fixed dentures held the highest share in the market, and are on the top of the list of many patients who prefer them to removable options due to the various benefits they offer. Compared to removable dentures, which are washable and can be taken off the mouth, fixed dentures are firmly attached to the existing teeth or dental implants, thus providing the patient with increased stability, better functionality, and a more natural feel. They not only make the dental structure look like the natural one, but also support the patient in the process of chewing and speech skills, creating a positive impact on the patient’s confidence and satisfaction level. In most cases, fixed dentures are made of durable porcelain or metal and are developed to be similar to real teeth in both shape and color. Fixed dentures are made to fit, alignment, and oral health benefits through the accurate examination and careful planning of dental professionals. Among the top reasons for fixed dentures to be chosen in Europe is the permanent stability that comes with these devices, thus, patients no longer have to worry about the shifting or slipping that usually occurs during daily activities, and therefore, they get more comfort, consistent functionality, and long-term oral health results.
Europe Dental Bone Graft Substitutes Market, By Material
The materials segment of the Europe dental graft substitutes market is divided into resins, metals, plastics, and other materials, of which resins are anticipated to record the highest CAGR throughout the forecast period. Resins are the most commonly selected materials in dental practice due to their compatibility with living tissues, long service life, and aesthetic versatility. Photopolymerizable polymers, among other high-performance resins, make it possible to accurately produce dentures with a superior fit and finish compared to conventional methods. At the same time, these materials include all the necessary characteristics of a dental product, such as strength, flexibility, and wear resistance, thus, they are a perfect choice for dental prosthetics that are guaranteed to provide patients' comfort for a long period. What is more, resins are light in weight, resistant to stains, and easy to care for, while their flexibility enables the dental professional to select the color of the natural teeth and thus achieve a highly aesthetic result. Besides, their capability to being directly bonded to tooth structures and allowing for exact shaping and polishing, thus, restoration durability, patient co mfort, and clinical efficiency are improved even further in contemporary dental practices in Europe.
EUROPE DENTAL BONE GRAFT SUBSTITUTES MARKET: COMPANY EVALUATION MATRIX
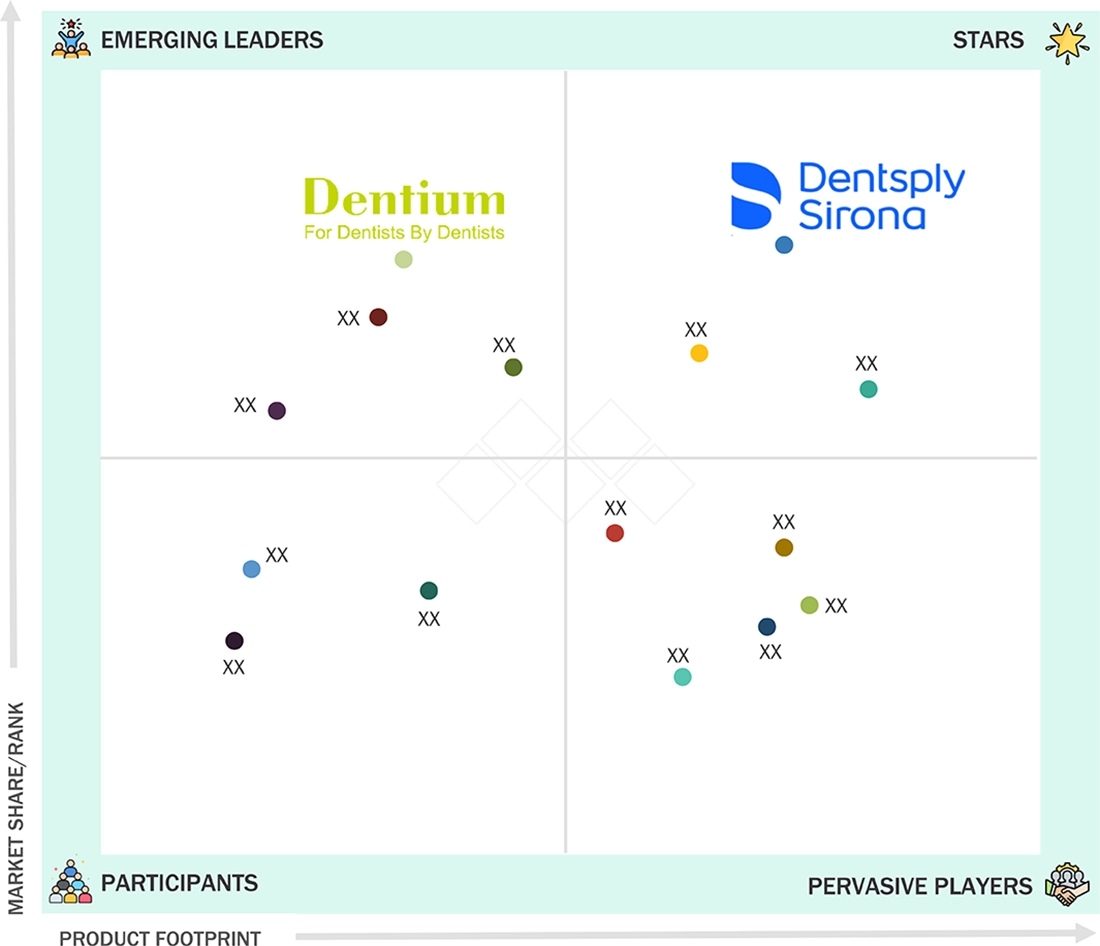
In the Europe dental bone graft substitutes market, Dentsply Sirona is positioned as a Star player, driven by its strong European presence, broad regenerative biomaterials portfolio, and established distribution network. Its integration of bone graft substitutes with implant and digital dentistry workflows, along with strong clinical acceptance, supports its leadership position. Dentium (South Korea) is viewed as an Emerging Player, gaining momentum in Europe through cost-effective bone graft solutions and expanding distributor partnerships. While Dentsply Sirona leads through scale and portfolio strength, Dentium’s competitive pricing and growing regional footprint position it for faster growth.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Dentsply Sirona (US)
- Institut Straumann AG (Switzerland)
- Kuraray Co. Ltd. (Japan)
- Johnson & Johnson (US)
- Stryker Corporation (US)
- Medtronic, Plc (Ireland)
- Zimvie Inc. (US)
- Henry Schein Inc. (US)
- Integra Lifesciences Holdings Corporation (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 411.1 Million |
| Market Size in 2031 (Value) | USD 641.5 Million |
| Growth Rate | CAGR of 6.4% from 2026 to 2031 |
| Years Considered | 2023–2031 |
| Base Year | 2025 |
| Forecast Period | 2026-2031 |
| Units Considered | Value (USD Million/Billion), Volume (Thousands Unit) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | By Type: Synthetic bone graft, Xenograft, Allograft, Autograft, Alloplast, Demineralized allograft, Others I By Product: Bio OSS, Osteograf, Grafton, Others I By Mechanism: Osteoconduction, Osteoinduction, Osteopromotion, Osteogenesis I By Application: Socket preservation, Ridge augmentation, Periodontal defect regeneration, Implant bone regeneration, Sinus lift I By End User: Hospitals Dental clinics, Other end users |
| Parent & Related Segment Reports | Dental Bone Graft Substitute Market |
WHAT IS IN IT FOR YOU: EUROPE DENTAL BONE GRAFT SUBSTITUTES MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Volume Analysis | Market assessment by volume (units) for dental equipment used in the digital dentures ecosystem |
|
| Company Information | Key players: Dentsply Sirona (US), Institut Straumann AG (Switzerland), Envista (US). Top 3–5 players market share analysis at the Asia Pacific and the North American country level | Insights on revenue shifts toward emerging innovations |
RECENT DEVELOPMENTS
- January 2024 : RTI Surgical (US) acquired Cook Biotech (US) to advance regenerative medicine and provide a broad range of allograft and xenograft biomaterials at competitive prices.
- November 2023 : Nobel Biocare (a subsidiary of Envista Holdings) launches its new product, creos syntogain, a biocompatible synthetic porous (bone) substitute with a multidirectional interconnected porous structure for use in periodontal, oral, and maxillofacial surgery.
Table of Contents

Methodology
The study involved four major activities in estimating the current size of the Europe dental bone graft substitute market. Exhaustive secondary research was done to collect information on the market, peer market, and parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the Europe dental bone graft substitute market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations operating in the Europe dental bone graft substitute market. The primary sources from the demand side included industry experts, purchase & sales managers, doctors, and personnel from research organizations. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
The research methodology used to estimate the size of the Europe dental bone graft substitute market includes the following details.
Country-level Analysis: The size of the Europe dental bone graft substitute market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products and services in the overall Europe dental bone graft substitute market was obtained from secondary data and validated by primary participants to arrive at the total dental bone graft substitute market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by end users and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall dental bone graft substitute market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes explained above—the market was split into several segments and sub-segments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
A dental bone graft substitute is a biocompatible material used by dentists to rebuild or augment jawbone tissue lost due to various reasons. These substitutes act as a scaffold or supportive structure to promote the growth of new bone tissue in the jaw. They essentially fill the defect or gap where bone loss has occurred.
Key Stakeholders
- Manufacturers and distributors of dental bone graft substitute
- Manufacturers and distributors of dental bone graft substitute components
- Dental bone graft substitute companies
- Healthcare institutes
- Diagnostic laboratories
- Dental Hospitals and clinics
- Academic institutes
- Research institutes
- Government associations
- Market research and consulting firms
- Venture capitalists and investors
Objectives of the Study
- To define, describe, segment, analyze, and forecast the Europe dental bone graft substitute market by type, application, mechanism, product, end user, and region.
- To provide detailed information about the factors influencing the market growth (drivers, restraints, opportunities, and challenges)
- To analyze micro markets concerning individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players.
- To profile the key players in the dental bone graft substitute market and comprehensively analyze their core competencies.
- To track and analyze competitive developments such as agreements, collaborations, and partnerships; expansions; acquisitions; and product launches and approvals in the Europe dental bone graft substitute market.
- To analyze the impact of the recession on the Europe dental bone graft substitute market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
Geographic Analysis
- Further breakdown of the Rest of Europe dental bone graft substitute market into Belgium, Russia, the Netherlands, Switzerland, and other countries.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Dental Bone Graft Substitutes Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Europe Dental Bone Graft Substitutes Market