
Europe Electrosurgery Market
Europe Electrosurgery Market By Product (Instruments, Accessories, Generators, Smoke Evacuation Systems), Surgery (Cardiovascular, Orthopedic, Cosmetic, Oncology, Urology), End User (Hospitals, Ambulatory Surgical Centers) - Regional Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
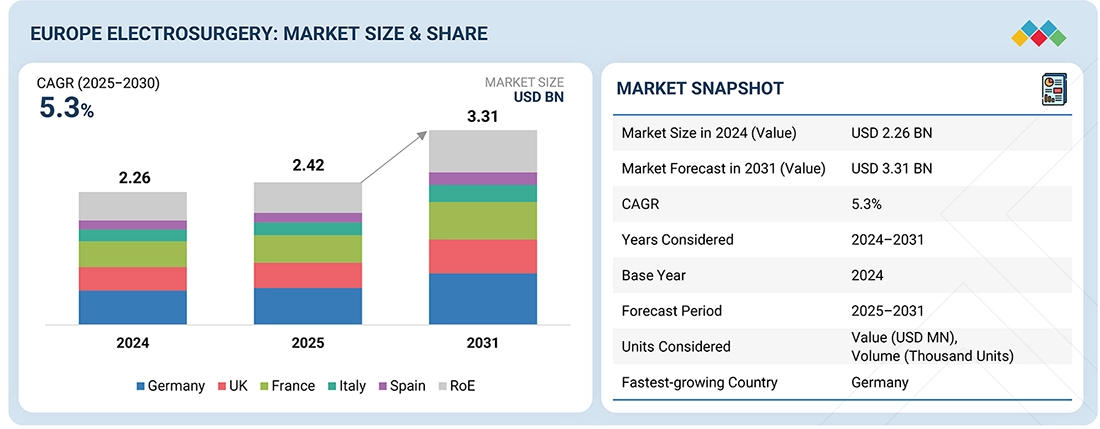
The Europe electrosurgery market, valued at USD 2.26 billion in 2024, stood at USD 2.42 billion in 2025 and is projected to advance at a resilient CAGR of 5.3% from 2025 to 2031, culminating in a forecasted valuation of USD 3.31 billion by the end of the period. The growth of the market is driven by the rising surgical volumes, an expanding elderly population, strong healthcare spending, and rapid adoption of advanced, safety-focused energy platforms across hospitals and specialty centers.
KEY TAKEAWAYS
-
By RegionGermany accounted for the largest share of 20-30% of the Europe electrosurgery market in 2024.
-
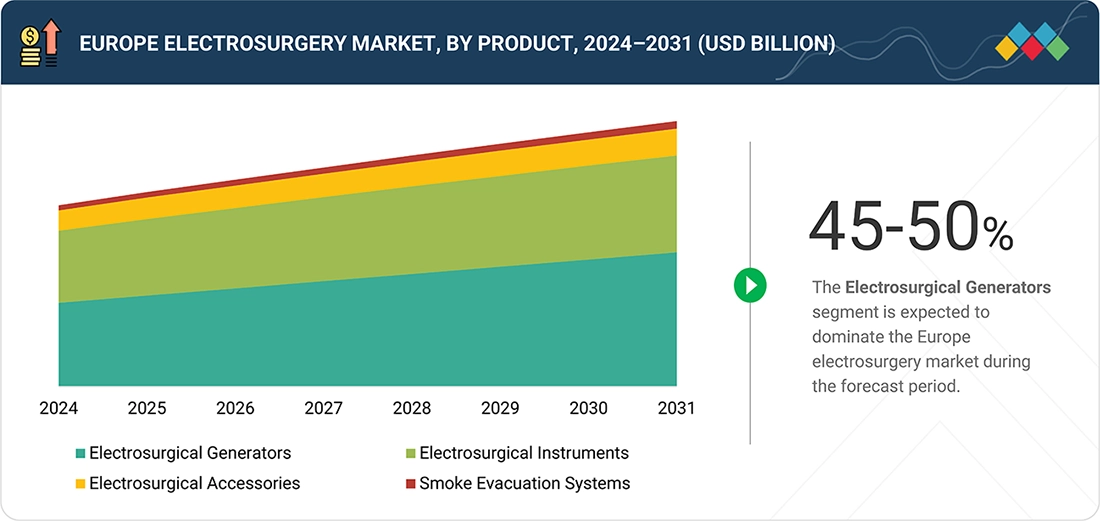
By ProductBased on product, the electrosurgical generators segment accounted for the largest share of 45-50% of the Europe electrosurgery market in 2024.
-
By SurgeryBased on surgery, the general surgery segment accounted for the largest share of 25-30% of the Europe electrosurgery market in 2024.
-
By End UserBased on end user, the hospitals, clinics & ablation centers segment held the largest share of 60-65% of the Europe electrosurgery market in 2024.
-
COMPETITIVE LANDSCAPEMedtronic (Ireland) and Johnson & Johnson (US) were recognized as star players due to their established, strong product portfolios.
-
COMPETITIVE LANDSCAPEAspen Surgical (US) (Progressive) and Bissinger Medizintechnik (Dynamic Companies) have established themselves among startups and SMEs, supported by strong product portfolios and effective business strategies.
The Europe electrosurgery market is influenced by an increasing number of minimally invasive procedures and the widespread adoption of advanced energy platforms. However, the market growth is also restrained by factors such as high device costs and stringent MDR requirements. The increasing government support for modernizing surgery offers significant opportunities, but the shortage of skilled healthcare professionals is challenging market growth.
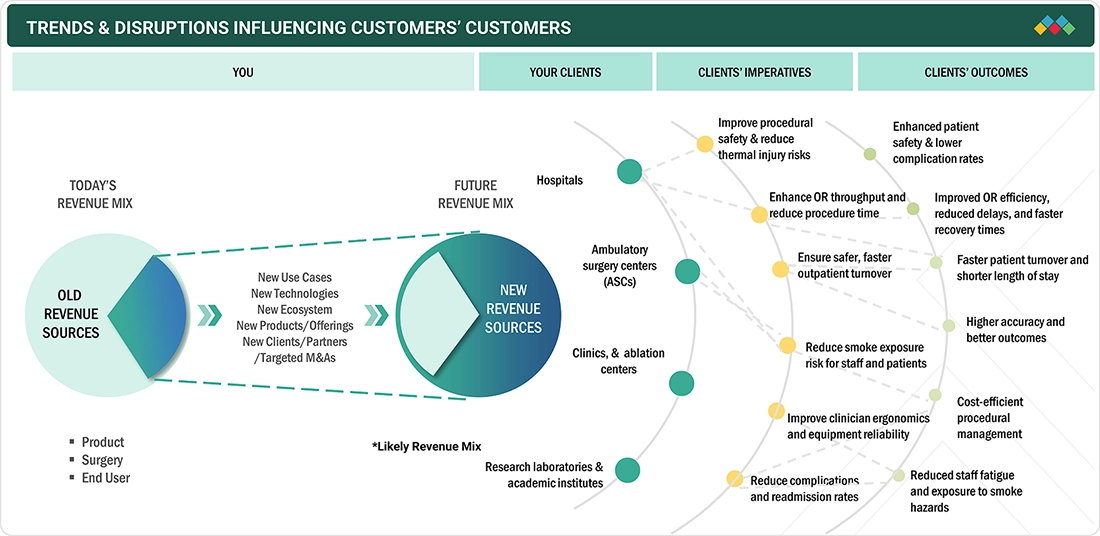
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The key trends and disruptions that affect the customer's customer, i.e., hospitals, surgeons, and surgical departments, include the accelerating shift toward minimally invasive procedures, which increases the demand for precision-driven electrosurgical platforms. In order to protect personnel from the hazards of surgical smoke, hospitals are being pushed to adopt integrated smoke-evacuation systems. Digital OR transformation, including data-enabled energy systems and device interoperability, is reshaping workflow expectations and purchasing decisions. Additionally, Europe’s aging population is driving higher volumes of orthopedic, oncologic, and cardiovascular surgeries. This is driving increased reliance on advanced electrosurgical products, such as electrosurgical generators and smoke evacuation systems. Disruptions such as MDR-driven compliance pressure and the transition to outpatient surgical models require suppliers to deliver smarter, safer, and cost-efficient products to stay relevant in clinicians’ evolving ecosystems.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing demand for minimally invasive surgeries

-
Innovation and technological advancements in electrosurgical instruments
Level
-
Risks associated with electrosurgical procedures
-
Stringent regulatory framework
Level
-
Rising government funding to develop advanced medical treatments
-
Expected increase in number of cosmetic and bariatric procedures due to growing obesity prevalence
Level
-
Concerns regarding toxic fumes produced during surgical procedures
-
Concerns about electromagnetic interference
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing number of hospitals coupled with growing number of surgical procedures
The increased number of hospitals across Europe, as well as the rise in surgical procedures, have been the main factors driving the growth of the Europe electrosurgery market. The expansion of healthcare infrastructure, particularly in Germany, France, Italy, and the Nordics, has increased procedural capacity and led to the adoption of advanced surgical technologies. The expansion is also supported by rising volumes of oncology, cardiovascular, orthopedic, gynecological, and bariatric surgeries, driven by an aging population and the increasing prevalence of chronic diseases. As hospitals upgrade operating rooms and adopt minimally invasive methods, the demand for electrosurgical generators, bipolar and monopolar instruments, and integrated smoke-management systems is increasing. Collectively, the expanding hospital network and escalating surgical load directly accelerate market growth.
Restraint: Risks associated with electrosurgical procedures
The growth of the Europe electrosurgery market is restrained by the risks inherently associated with electrosurgical procedures, which influence both clinician adoption and regulatory scrutiny. Potential complications, including inadvertent thermal injury, insulation failure, burns, stray-current damage, and risks from surgical smoke exposure, create caution among healthcare providers and necessitate stringent safety protocols. These risks also drive hospitals to invest in extensive staff training, device validation, and compliance with MDR requirements, increasing operational burdens. Furthermore, incidents linked to improper device use or equipment malfunction can lead to litigation concerns and slower procurement decisions. As a result, despite technological advancements, safety-related apprehensions continue to moderate the pace of electrosurgical adoption across Europe.
Opportunity: Expected increase in number of cosmetic and bariatric procedures due to growing obesity prevalence
The escalating obesity rates throughout Europe represent a major opportunity for the electrosurgery market, as it is the main factor that contributes to the demand for both cosmetic and bariatric procedures. Weight-loss surgeries like gastric bypass, sleeve gastrectomy, and other minimally invasive procedures are increasingly performed, and they require cutting, coagulation, and vessel-sealing technologies, which are the main applications of electrosurgical devices. At the same time, the trend towards cosmetic procedures such as body contouring and aesthetic enhancers is leading to more procedures being performed in hospitals and private clinics. With the increase in patient awareness and the support of healthcare systems for treatments related to obesity, manufacturers of advanced electrosurgical generators, bipolar instruments, and energy platforms are well-positioned to capture accelerated growth through targeted product innovation and clinical partnerships.
Challenge: Concerns regarding toxic fumes produced during surgical procedures
One of the major issues limiting the development of the Europe electrosurgery market is the concern over the release of toxic fumes during electrosurgical procedures. Surgical smoke is a source of many harmful chemicals, particulate matter, and bioaerosols, which can worsen air quality and cause health problems for surgeons, nurses, and other operating room staff. The increased awareness of such risks has led to intensified scrutiny by regulatory authorities and hospital safety committees, forcing healthcare facilities to install surgical smoke evacuation systems and to improve OR infrastructure in compliance with safety standards. Nevertheless, quite a few hospitals are struggling with financial issues and are finding it difficult to integrate the different systems, and their work is interrupted when they decide to implement new technologies to manage smoke. These operational and financial challenges can lead to delays in purchasing decisions, slow adoption of electrosurgical devices, and hesitancy among end users, ultimately challenging market expansion despite technological advancements.
EUROPE ELECTROSURGERY MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Offers Valleylab energy platforms, HET bipolar systems, electrosurgical electrodes, and a broad range of monopolar/bipolar instruments designed for general, bariatric, colorectal, and gynecological surgeries | Systems feature adaptive energy delivery for real-time tissue feedback and integrated smoke management capability | Enhances safety through intelligent tissue sensing, reduces complications linked to overheating, and optimizes cutting/coagulation performance | Supports efficient OR workflows, lowers surgical-smoke exposure, and improves consistency across diverse surgical specialties |
 |
Provides Gynecare VersaPoint bipolar systems, Endopath Probe Plus, electrosurgical accessories, electrodes, and smoke evacuation systems designed for minimally invasive gynecological, general, and urological surgeries | Devices enable controlled desiccation, resection, and vessel sealing with reduced collateral tissue damage | Improves surgical safety with lower thermal risk, delivers precise energy control, and enhances procedural outcomes in delicate anatomical areas | Smoke evacuation enhances air quality and reduces staff fatigue, while reliable bipolar solutions support minimally invasive adoption |
 |
Provides advanced hybrid-energy devices (THUNDERBEAT), SurgMaster and ESG electrosurgical generators, and smoke management systems designed for laparoscopic, open, and specialty procedures | Systems enable multifunctional dissection, precise vessel sealing, and optimized OR integration with enhanced visibility and safety | Improves surgical precision and procedural efficiency, reduces thermal spread, enhances visibility through advanced smoke evacuation, and shortens procedure time| Supports minimally invasive workflows and delivers consistent tissue effects, improving patient outcomes and surgeon ergonomics |
 |
Offers monopolar and bipolar accessories, bipolar electrosurgery units, and specialized electrodes engineered for general, endoscopic, and specialty surgical procedures | Designed for reproducible energy output, user safety, and compatibility with European OR standards | Reduces unintended tissue injury through controlled power delivery, enhances procedural reliability, and supports MDR-compliant OR environments | Improves surgeon confidence, minimizes equipment-related risks, and enables consistent results across high-volume surgical centers |
 |
Provides electrosurgical generators, including System 5000 ESU, System 2450 ESU, and Sabre Genesis ESU, along with a full suite of electrosurgical instruments and smoke-management solutions | Systems optimized for general, laparoscopic, orthopedic, and gynecologic procedures requiring stable, high-frequency energy | Delivers stable energy performance, enhances precision, and improves OR safety via effective smoke evacuation | Enables high-efficiency workflows, reduces procedural delays, and minimizes thermal injury risks for surgeons and patients |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The ecosystem of the Europe electrosurgery market is an interconnected network of electrosurgery product manufacturers, regulatory bodies, healthcare providers, distributors, and end users. Top OEMs produce electrosurgical generators, instruments, accessories, and smoke-evacuation systems that comply with strict MDR requirements and hospital safety standards. Distributors and group purchasing organizations enable procurement to run smoothly between public and private hospitals, clinics, ASCs, ablation centers, and other end users. Healthcare providers, surgeons, OR managers, and biomedical engineers are the main decision-makers. Thus, they have the greatest influence on product selection based on the product performance, safety, interoperability, and cost-effectiveness. Regulatory bodies help with compliance, certification, and the adoption of best practices. Research institutes and academic centers support clinician skill development, while patients drive demand through rising surgical volumes. Collectively, this ecosystem enables technology innovation, commercialization, and sustained market growth.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Electrosurgery Market, By Product
The electrosurgical generators segment dominated the Europe electrosurgery market in 2024. They are the main energy source for all surgical energy applications. The increasing procedure volumes caused by aging and obese populations and the fast uptake of advanced, safety-integrated platforms facilitate minimally invasive and high-precision surgical workflows both in hospitals and clinics.
Europe Electrosurgery Market, By Surgery
The general surgery segment accounted for the largest share of the Europe electrosurgery market in 2024. It encompasses high-volume procedures, such as bariatric, abdominal, vascular, endocrine, and soft-tissue surgeries. The growth of this segment is driven by an aging population and expanding surgical center capacity.
Europe Electrosurgery Market, By End User
Hospitals, clinics, and ablation centers dominated the Europe electrosurgery market in 2024, as they perform the highest volume of complex and routine surgeries. The market in this segment is also supported by the availability of skilled healthcare professionals, advanced OR infrastructure, and strong reimbursement systems.
REGION
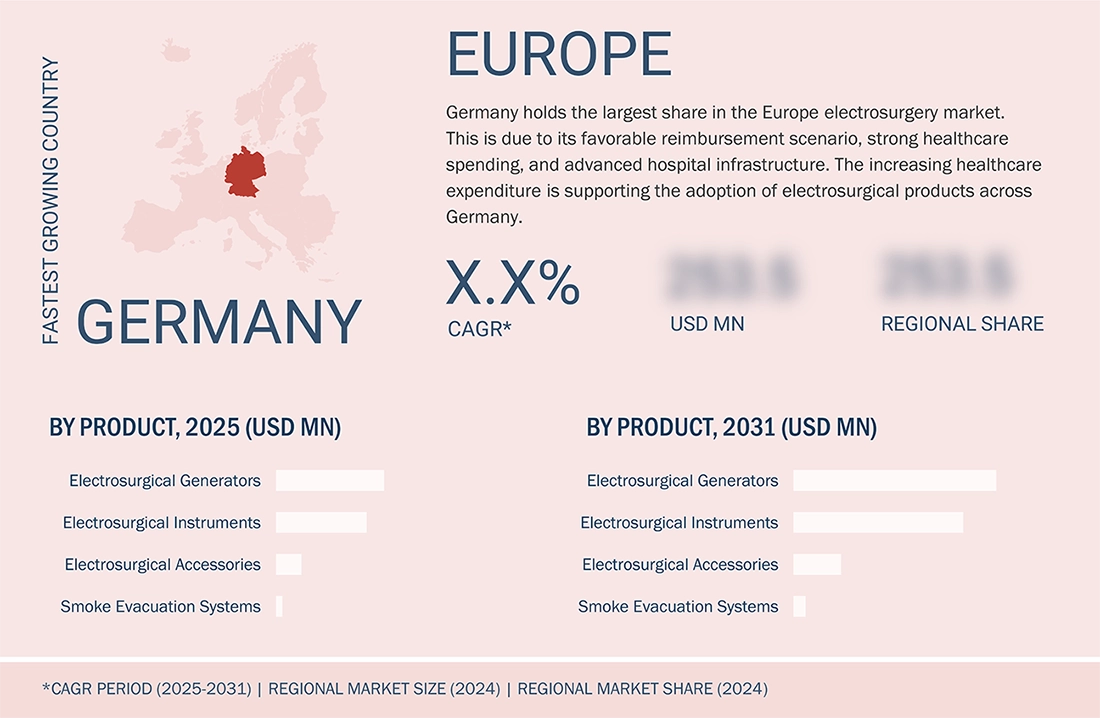
Germany held the largest share in Europe Electrosurgery market in 2024.
Germany holds the largest share of the Europe electrosurgery market. This is due to its favorable reimbursement scenario, strong healthcare spending, and advanced hospital infrastructure. The increasing healthcare expenditure is supporting the adoption of electrosurgical products across Germany. According to Eurostat, Germany spent USD 533.8 billion in 2023, equivalent to 13.2% of its GDP. German hospitals are well-positioned to invest in advanced electrosurgical products. Statutory Health Insurance (SHI) further reduces financial barriers for surgical procedures, driving high procedure volumes across general, cardiovascular, orthopedic, and oncological surgeries. Combined with a large and growing elderly population and the presence of leading domestic manufacturers, Germany leads the market in Europe.
EUROPE ELECTROSURGERY MARKET: COMPANY EVALUATION MATRIX
In the Europe electrosurgery market matrix, Medtronic (Ireland) (Star) and Olympus Corporation (Japan) (Star) lead with their unmatched global presence, strong brand recognition, and comprehensive portfolios of electrosurgery products. Smith+Nephews (UK) (Emerging Leader) is rapidly gaining traction with its versatile products, which offer electrosurgery products for various applications such as general surgery, cosmetic surgery, and cardiovascular surgery.
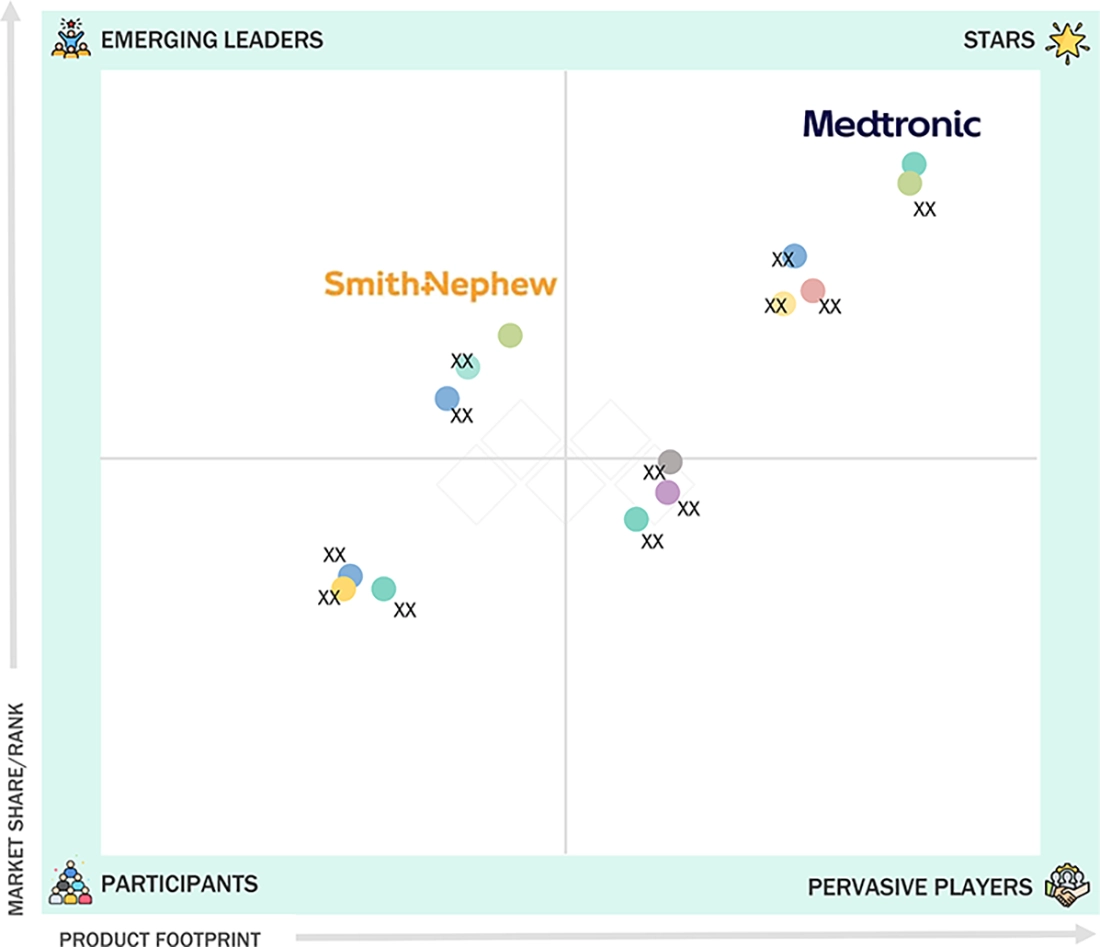
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Medtronic
- Johnson & Johnson
- Olympus Corporation
- B Braun
- Conmed Corporation
- Boston Scientific
- Smith+Nephew
- Erbe Ellectromedizin
- Stryker Corporation
- Bowa Electronic GmbH & Co. kg
- Zimmer Biomet
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size Value in 2025 (USD Billion) | 2.42 |
| Revenue Forecast in 2031 (USD Billion) | 3.31 |
| Growth Rate | CAGR of 5.3% from 2025-2031 |
| Years Considered | 2024-2031 |
| Base Year | 2024 |
| Forecast Period | 2025-2031 |
| Units considered | Value (USD Million), Volume (Thousand Units) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
| Regional Scope | Germany, UK, Italy, France, Spain, Rest of Europe |
WHAT IS IN IT FOR YOU: EUROPE ELECTROSURGERY MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Company Information | Market share analysis by country (Germany and UK) & competitive leadership mapping for established players in the market | Insights on market share analysis by country |
| Geographic Analysis | Further breakdown of the Rest of Europe market into Russia, Switzerland, Denmark, Austria, and others | Country-level demand mapping for new product launches and localization strategy planning |
RECENT DEVELOPMENTS
- October 2025 : Olympus Corporation announced the launch of THUNDERBEAT II, its next-generation hybrid energy device designed for advanced soft-tissue management across Europe, the Middle East, and Africa (EMEA).
- April 2025 : Erbe Elektromedizin GmbH introduced its next-generation tailored electrosurgical generators designed to optimize high-performance surgical workflows. The launch features VIO® 3n and VIO® seal, two advanced systems that elevate electrosurgical precision, efficiency, and procedural safety.
- February 2025 : BOWA MEDICAL UK announced a key milestone in the development of its new headquarters in Kingskerswell, signaling progress in the company’s long-term expansion strategy. The upcoming facility is expected to substantially strengthen BOWA’s operational capacity and enable the company to better address the rising demand for its electrosurgical products across the UK market.
- May 2024 : Erbe Elektromedizin GmbH inaugurated a state-of-the-art production and development facility in Rangendingen, near Tübingen, Germany. The 25,000 m² plant significantly enhances the company’s global manufacturing capacity for advanced medical technology instruments.
Table of Contents

Methodology
This research study involved the extensive use of both primary and secondary sources. It involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, the competitive landscape of market players, and key market dynamics such as drivers, opportunities, challenges, restraints, and key player strategies.
Secondary Research
This research study involved the wide use of secondary sources, directories, databases such as Dun & Bradstreet, Bloomberg Businessweek, and Factiva, white papers, and companies’ house documents. Secondary research was undertaken to identify and collect information for this extensive, technical, market-oriented, and commercial study of the Europe electrosurgery market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various supply side and demand side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, engineers, and related key executives from various companies and organizations operating in the Europe electrosurgery market. Primary sources from the demand side included hospitals, clinics, researchers, lab technicians, purchase managers etc, and stakeholders in corporate & government bodies.
Market Size Estimation
The market size for Europe electrosurgery market was calculated using data from four different sources, as will be discussed below. Each technique concluded and a weighted average of the four ways was calculated based on the number of assumptions each approach made. The market size for Europe electrosurgery market was calculated using data from four distinct sources, as will be discussed below:
Data Triangulation
The entire market was split up into several segments when the market size was determined. Data triangulation and market breakdown processes were used where necessary to complete the entire market engineering process and arrive at the precise statistics for all segments.
Approach to derive the market size and estimate market growth.
Using secondary data from both paid and unpaid sources, the market rankings for the major players were determined following a thorough analysis of their sales of electrosurgery. Due to data restrictions, the revenue share in certain cases was determined after a thorough analysis of the product portfolio of big corporations and their individual sales performance. This information was verified at each stage by in-depth interviews with professionals in the field.
Market Definition
Electrosurgery is a surgical technique that uses high-frequency electrical currents to cut, coagulate, or remove tissue during procedures. It offers precise tissue control, reduced blood loss, and shorter recovery times, making it widely used across medical specialties for minimally invasive surgeries.
Key Stakeholders
- Electrosurgical product manufacturing companies
- Distributors, suppliers, and commercial service providers
- Healthcare service providers
- Contract research organizations (CROs)
- Medical research laboratories
- Academic medical centers and universities
- Market research and consulting firms
- Hospitals and clinics
- Cancer care and ablation centers
- Ambulatory surgery centers (ASCs)
- Research laboratories
Objectives of the Study
- To define, describe, and forecast the Europe electrosurgery market based on by product, surgery type, end user, and region
- To provide detailed information about the major factors influencing the market growth (such as drivers, restraints, challenges, and opportunities)
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall Europe electrosurgery market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To profile the key players and comprehensively analyze their market shares and core competencies in the Europe electrosurgery market
- To track and analyze competitive developments such as partnerships, expansions, acquisitions, collaborations, service launches, agreements, and other developments in the Europe electrosurgery market
- To benchmark players within the Europe electrosurgery market using the Company Evaluation Quadrant framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and service offerings
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Geographic Analysis
- Further breakdown of the Rest of Europe Electrosurgery Market into Denmark, Norway, and others
- Further breakdown of the Rest of Asia Pacific Electrosurgery Market into Vietnam, New Zealand, and others
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Electrosurgery Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Europe Electrosurgery Market