
Europe Leukapheresis Market Size, Growth, Share & Trends Analysis
Europe Leukapheresis Market by Product (Devices, Disposable, Membrane Separators), Leukopak (Mobilized, Non-Mobilized), Indication (ALL, NHL, Multiple Myeloma), Application (Research, Therapeutic), End User (Hospitals, Pharma, Biotech) - Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
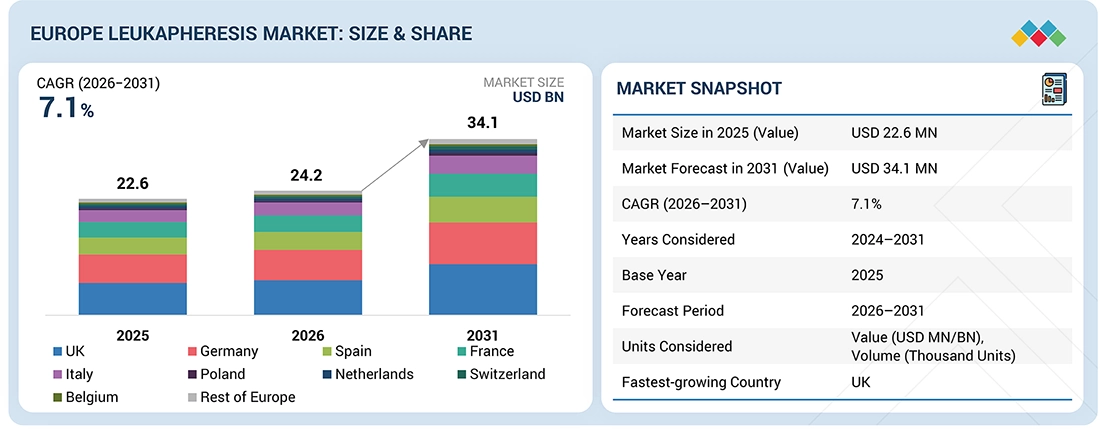
The Europe Leukapheresis market, valued at USD 22.6 million in 2025, stood at USD 24.2 million in 2026 and is projected to advance at a resilient CAGR of 7.1% from 2026 to 2031, culminating in a forecasted valuation of USD 34.1 million by the end of the period. This growth is due to the increasing number of applications for leukapheresis in cases pertaining to hematological disorders, coupled with an increase in the number of starting materials for cell & gene therapy applications. Furthermore, there has been significant development in apheresis facilities in key countries in the region.
KEY TAKEAWAYS
-
Europe Leukapheresis Market, By TypeIn the Europe leukapheresis market, the disposables segment accounted for the largest market share of 77.4% in 2025.
-
Europe Leukapheresis Market, By ApplicationResearch applications segment is projected to grow at the highest CAGR of 7.2% during the forecast period in the Europe leukapheresis market.
-
Europe Leukapheresis Market, By End UserIn the Europe leukapheresis market, the blood component providers & blood centers segment held the largest market share of 45.8% in 2025.
-
Europe Leukapheresis Market, By CountryUK is projected to grow at the highest CAGR of 7.2% during the forecast period, in the Europe leukapheresis market.
-
Europe Leukopaks Market, By TypeBy type, the mobilized leukopaks segment dominated the Europe leukopaks market by market share in 2025.
-
Europe Leukapaks Market, By IndicationBy indication, the hepatocellular carcinoma segment is projected to register the highest CAGR of 27.4% during the forecast period.
-
Europe Leukapaks Market, By End UserIn Europe leukopaks market, the academic & research institutes segment captured a 63.3% market share in 2025.
-
Europe Leukopaks Market, By CountryIn Europe leukopaks market, the UK is expected to witness the fastest growth rate of 24.2% from 2026 to 2031.
-
COMPETITIVE LANDSCAPE - MAJOR PLAYERSTerumo BCT (Japan) and Fresenius Kabi (Germany) are identified as star performers in the Europe leukapheresis market, as they have a very strong installation base, sophisticated leukapheresis technologies, and broad adoption of these technologies in European hospitals & blood banks.
-
COMPETITIVE LANDSCAPE - STARTUP/SMEImmune Therapy Holdings AB (Sweden) and SCTbio (Czech Republic) have been identified as progressive SMEs, as they have been growing rapidly in cell processing & cell therapy services.
The leukapheresis market in Europe is a niche but steadily expanding segment, supported by advanced hospital infrastructure and growing adoption in hematology, oncology, and cell and gene therapy workflows. Demand is concentrated in Western Europe, where established apheresis centers, rising CAR-T activity, and favorable reimbursement environments continue to support consistent market growth.
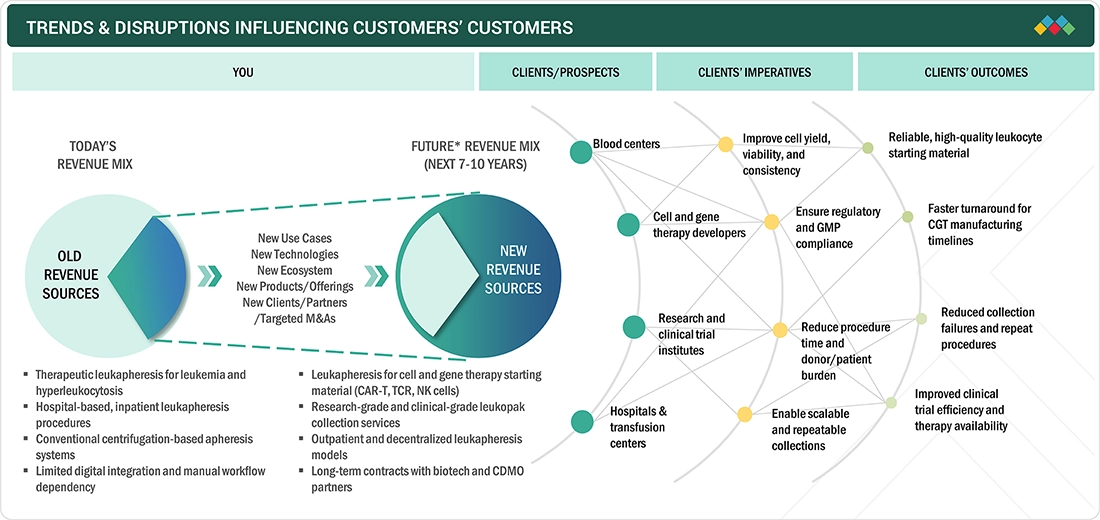
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Europe leukapheresis market is facing a fundamental change due to the evolving trend of the need for the process, which is shifting from being acute, clinic-driven, and therapeutic to being routine for cell therapies. The need for high-quality, reliable, and fully GMP-compliant processes for CAR-T, advanced immunotherapies boosts the need for better processes because of growth in these technologies. Digitalization, outpatient care, and Pan Europe collections are changing the service dynamic.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising incidence of leukemia

-
Increasing blood donation
Level
-
High cost of therapeutic leukapheresis and leukopaks
-
Stringent donor recruitment criteria
Level
-
Growing focus on leukapheresis for pediatric patients
-
Increasing investments in CAR-T therapies
Level
-
Concerns related to safety of blood transfusion in emerging economies
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising incidence of leukemia
The main reasons driving the Europe leukapheresis market are the rise in the incidence of leukemia across the region, especially among aging populations in Germany, France, Italy, and the UK. Leukapheresis sees broad applications both as a therapeutic intervention aimed at a rapid reduction in high white blood cell counts and as a preparatory step for advanced treatments. Further helping to sustain demand across the region is a strong network of hematology care, early diagnosis, and wider access to specialized oncology treatment centers.
Restraint: High cost of therapeutic leukapheresis and leukopaks
The cost involved in leukapheresis procedures and leukopak processing hinders the use of the technique to a larger extent, especially outside Western Europe. This increases the total cost of treatment, considering the other costs involved, including sophisticated equipment used in apheresis, disposables, skilled laboratory personnel, and GMP processing. A number of countries with limited budgets and delayed reimbursement approvals may result in reduced processing, hence hampering the growth of the market.
Opportunity: Growing focus on leukapheresis for pediatric patients
Europe is witnessing increasing traction in leukapheresis performed for pediatric use, owing to the rise of survival rates in childhood leukemia and the expanding application of cell and gene therapies in pediatric oncology. Specialized children’s hospitals and research centers are increasingly adopting pediatric-friendly apheresis protocols in Western Europe. Ongoing clinical trials and supportive regulatory environment that foster innovative therapies open up opportunities for leukapheresis services designed to suit younger patient populations.
Challenge: Concerns related to safety of blood transfusion in emerging economies
Blood safety concerns linked to handling and processing outside the body have been an area that is yet to be overcome by the leukapheresis market in Europe. Tough policies and norms framed by the EU in relation to blood safety and traceability have been posing hurdles in adopting them. Blood-related concerns linked to infection risks and anticoagulant-related adverse events have always been at the forefront.
EUROPE LEUKAPHERESIS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Provides leukapheresis systems and disposables widely used in European hospitals for therapeutic leukocyte reduction and standardized cell collection supporting hematology and oncology workflows | Strong reliability | Broad hospital penetration in Europe | Integrated consumables portfolio | Compliance with EU clinical and safety standards ensure consistent procedural outcomes |
 |
Supplies automated apheresis platforms used across Europe for efficient leukocyte separation in therapeutic leukapheresis and immune cell collection for advanced clinical applications | Improved cell yield consistency | Reduced procedure times | Enhanced patient safety | Support for scalable adoption in busy European apheresis centers |
 |
Delivers continuous-flow leukapheresis systems used for both therapeutic indications and collection of starting material for cell and gene therapies in European treatment centers | Precise cell separation | Flexible protocols | Strong support for CGT workflows help European providers achieve high-quality collections and operational efficiency |
 |
Offers apheresis disposables and blood processing solutions supporting leukapheresis procedures within European hospitals and transfusion services | Cost-effective consumables | Strong regional presence | Alignment with European blood safety regulations support reliable and compliant leukapheresis operations |
 |
Provides leukapheresis-related systems and blood management solutions used in therapeutic leukocyte reduction and research-grade cell collection across Europe | Deep expertise in blood therapies | Strong transfusion network integration | Regulatory experience enable safe procedures and dependable access to leukocyte products |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe leukapheresis market is intertwined with apheresis systems manufacturers, disposables suppliers, hospitals, blood banks, and cell processing units. These participants collaborate with government agencies, research institutions, and cell and gene therapy companies to provide a safe environment for cell harvesting. Developed public healthcare infrastructure and a biotech environment make a positive impact on adopting this procedure coveringly around Europe.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
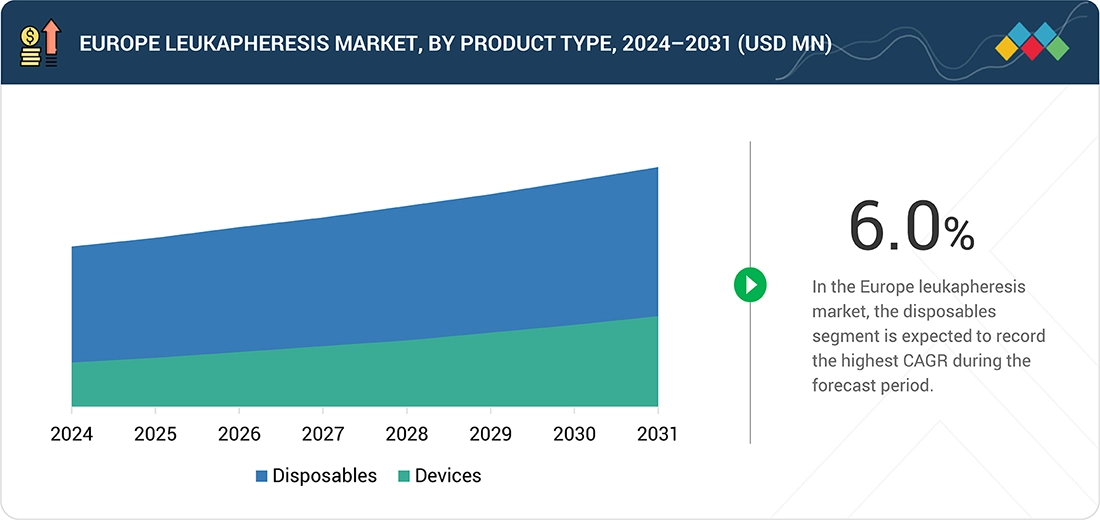
Europe Leukapheresis Market, By Type
The Europe leukapheresis market is dominated by the disposables segment as single-use sets, kits, and filters are used in every leukapheresis procedure. Strict regulations in the European market for infectious control and blood safety promote the use of disposable parts over reusable parts. The continually increasing number of leukapheresis procedures in the European market ensures the disposables segment is a leading contributor to the market.
Europe Leukapheresis Market, By Application
Research applications segment is expected to register the highest CAGR during the forecast period due to continued development of the research ecosystem for cell and gene therapy within the European region. With the rise in number of clinical trials involving CAR-T, TCR, and other immunotherapies, there has been a rising need for cells derived from the process of leukapheresis.
Europe Leukapheresis Market, By End User
Providers of blood components and blood centers occupy the leading market position in Europe due to their critical role in organizing and standardizing processes related to leukapheresis. Blood centers have established infrastructure and a trained workforce that is necessary for a large-scale procedure of obtaining components from blood and are able to undertake a large number of processes related to clinical trials and use of devices and consumables of leukapheresis.
Europe Leukopaks Market, By Type
The mobilized leukopak segment in Europe holds a major market share as mobilized leukopaks are known to give a higher yield of target immune cells required for R&D in advanced research. The process of mobilization in research institutions and clinical trial settings is quite prominent because of its contribution towards steady cell availability. The complexity of research in immunotherapy further fuels the demand for mobilized leukopak.
Europe Leukopaks Market, By Indication
The hepatocellular carcinoma segment is anticipated to record the highest CAGR in the Europe leukopaks market, particularly due to the rising cases of liver cancer. Moreover, immuno-oncology studies are extensively being carried out in European research institutions for the treatment of liver cancer. In turn, this has led to an increased demand for research leukopaks.
Europe Leukopaks Market, By End User
The Europe leukopaks market is dominated by research and academic institutes as they are major users of leukopaks in immunology, oncology, and translational research. Adequate public funding, developed research infrastructure, and engagement in global clinical trials drive high demand volumes. The involvement of research institutes in early-stage therapy R&D and validation creates steady demand in the Europe leukopaks market.
REGION
UK to be fastest-growing in Europe leukapheresis market during forecast period
The UK is likely to report the highest CAGR in the Europe leukapheresis market due to its robust research environment and the adoption of innovative cell and gene therapies. Rising approvals of CAR-T cell therapies, an increasing number of clinical trials, and an established apheresis procedure environment in NHS hospitals are fueling procedure numbers. Favorable regulations and an increasing trend of collaboration among academia, biotechnology companies, and blood establishments quickly move the Europe leukapheresis market forward.
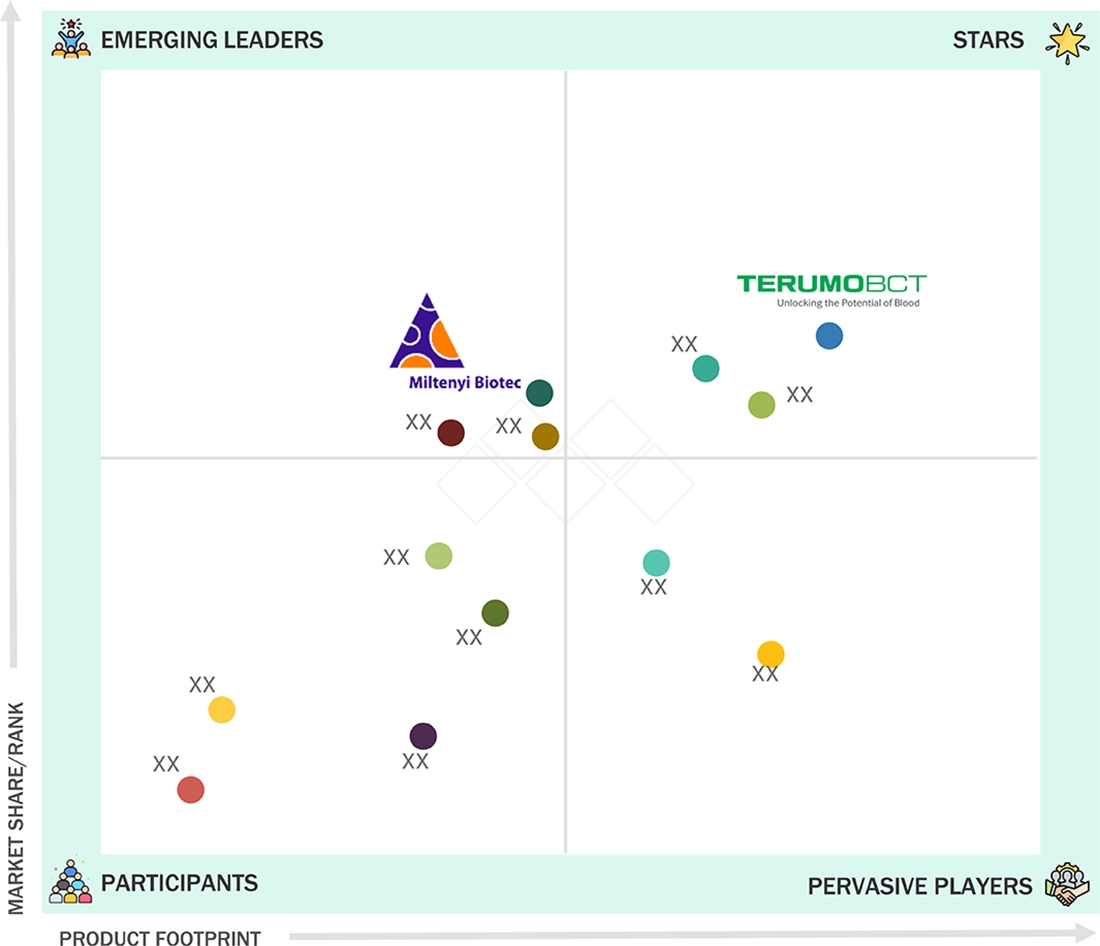
EUROPE LEUKAPHERESIS MARKET: COMPANY EVALUATION MATRIX
Terumo BCT is categorized under the star players for the Europe leukapheresis market as the company has an installed base of leukapheresis systems, has been innovative with their products, and has penetrated hospitals and blood banks extensively. Miltenyi Biotec is an emerging leader for the Europe leukapheresis market as the focus of the company has been shifting to cell and gene therapy, which has helped the company gain prominence in the market.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Fresenius Kabi (Germany)
- Terumo BCT (Japan)
- Haemonetics Corporation (US)
- Grifols S.A. (Spain)
- Macopharma (France)
- Medica S.p.A. (Italy)
- Miltenyi Biotec (Germany)
- Baxter International Inc. (US)
- Nikkiso Co., Ltd. (Japan)
- Immune Therapy Holdings AB (Sweden)
- SCTbio (Czech Republic)
- Biohope Scientific SL (Spain)
- Caltag Medsystems Ltd. (UK)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 22.6 MN |
| Market Forecast in 2031 (Value) | USD 34.1 MN |
| CAGR (2026-2031) | 7.1% |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD MN/BN), Volume (Thousand Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, the UK, France, Italy, Spain, Poland, the Netherlands, Switzerland, Belgium, and Rest of Europe |
| Parent & Related Segment Reports | Leukapheresis Market |
WHAT IS IN IT FOR YOU: EUROPE LEUKAPHERESIS MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Detailed assessment of leukapheresis systems and disposables by procedure type (therapeutic vs. collection), application (clinical vs. research), and technology (continuous-flow, automation level). | Identified adoption trends in CGT-driven leukapheresis, increased use of single-use disposables, and growing demand for standardized, GMP-aligned collection workflows across Europe. |
| Company Information | Comprehensive profiles of key leukapheresis players such as Fresenius Kabi, Terumo BCT, Haemonetics, Grifols, and emerging European SMEs. | Mapped competitive positioning, product portfolios, partnerships, training initiatives, and strategic moves supporting cell and gene therapy expansion in the Europe leukapheresis market. |
| Geographic Analysis | Delivered country-level analysis of the Europe leukapheresis market covering Germany, France, UK, Italy, Spain, and emerging markets. Assessed regulatory landscape (EMA, national agencies), healthcare infrastructure, and CGT activity. | Supported strategic planning by identifying high-growth countries, strong apheresis hubs, favorable reimbursement environments, and opportunities for cross-border collection networks and partnerships. |
RECENT DEVELOPMENTS
- August 2023 : Fresenius Kabi (Germany) and Lupagen Inc. (US) entered a strategic development and supply agreement focused on advancing technologies for cell and gene therapy delivery. The collaboration aims to accelerate translation of advanced therapies from development to bedside use.
- May 2023 : Terumo BCT (US) introduced a specialized training program to help cell and gene therapy manufacturers optimize cell collection processes. The initiative supports faster scale-up and commercialization of advanced therapeutics through improved leukapheresis practices.
- April 2023 : Fresenius Kabi (Germany) upgraded its Amicus Blue ECP system with new software versions and a flexible single-use disposable kit. This enhances procedural efficiency, usability, and venous access options for leukapheresis and photopheresis workflows.
- January 2023 : Charles River Laboratories International, Inc. (US) launched its CliniPrime Fresh Leukopak offering, providing GMP-compliant cellular starting materials. This supports cell and gene therapy developers with high-quality leukopaks suitable for clinical and commercial manufacturing.
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, key players, competitive landscape, key market dynamics, and key player strategies.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the Europe leukapheresis market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations operating in the Europe leukapheresis market. The primary sources from the demand side included industry experts, purchase & sales managers, doctors, and personnel from research organizations. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The total size of the Europe leukapheresis market was arrived at after data triangulation from three different approaches, as mentioned below. After each approach, the weighted average of the three approaches was taken based on the level of assumptions used in each approach. The same approach was used for the leukopaks market.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes explained above—the market was split into several segments and sub-segments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Leukapheresis is the process of separating white blood cells from the whole blood. Therapeutic leukapheresis procedures are used to treat hyperleukocytosis leukemia, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), multiple myeloma, and non-Hodgkin’s lymphoma.
Leukopaks are enriched products that contain mononuclear leukocytes (lymphocytes and monocytes) and are used to develop cell-based therapeutics.
Key Stakeholders
- Apheresis Device Manufacturers and Distributors
- Europe Leukapheresis Product Manufacturers and Distributors
- Leukopak Manufacturers and Distributors
- Pharmaceutical and Biotechnology Companies
- Healthcare Service Providers (including Hospitals and Transfusion Centers)
- Cancer Treatment Centers
- Blood Component Providers & Blood Centers
- Contract Research Organizations (CROS)
- Academic and Research Institutes
- Government Associations
- Market Research and Consulting Firms
- Venture Capitalists and Investors
Objectives of the Study
- To define, describe, and forecast the Europe leukapheresis products market by type, application, end-user, and region
- To define, describe, and forecast the leukopaks market by type, indication, end-user, and region
- To strategically analyze the industry trends, technology trends, pricing analysis, regulatory scenario, supply/value chain, ecosystem/market map, Porter’s Five Forces, trade & patent analysis, key stakeholders & buying criteria, and conferences & events
- To provide detailed information about the factors influencing market growth (drivers, restraints, opportunities, and challenges)
- To analyze micro markets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To strategically profile key players in the Europe leukapheresis market and comprehensively analyze their core competencies
- To track and analyze competitive developments such as acquisitions, product launches, approvals, expansions, and partnerships
- To analyze the impact of the recession on the Europe leukapheresis market
Available customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Geographic Analysis
- Further breakdown of the RoE leukapheresis market into Austria, Finland, and others
- Further breakdown of the RoLATAM leukapheresis market into Brazil, Mexico, Argentina, Colombia, Chile, and others
Competitive Landscape Assessment
- Market share analysis for the North America and Europe region, which provides market shares of the top 3–5 key players in the Europe leukapheresis market
- Competitive leadership mapping for established players in the US
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Leukapheresis Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Europe Leukapheresis Market