
Europe Pharmaceutical Packaging Market
Europe Pharmaceutical Packaging Market by Raw Material (Plastic, Paper & Paperboard, Glass, Metal), Type (Plastic Bottles, Blisters, Caps & Closures, Labels & Accessories, Prefilled Syringes), Drug Delivery, and Region - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
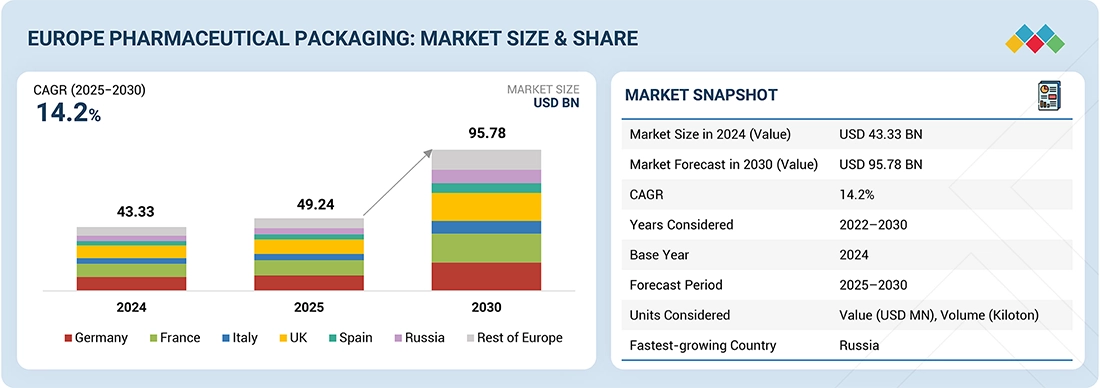
The Europe pharmaceutical packaging market is projected to reach USD 95.78 billion by 2030 from USD 49.24 billion in 2025, at a CAGR of 14.2% during the forecast period. The Europe Pharmaceutical Packaging reports that growth is driven by demand for packaging that is safe, convenient, and sustainable through advances in various technologies. An elevated level of regulatory compliance regarding product safety and efficacy demands that packaging technology manufacturers provide solutions through innovative products and services. Examples include, but are not limited to, Pre-filled Syringes, child-resistant closures (CRCs), Tamper-Evident Packaging Formats (TEPF), and Smart Packaging. Manufacturers will provide new opportunities for growth to pharmaceutical firms that use sustainable and recyclable materials by providing manufacturers with an opportunity to continue to meet their global sustainability goals by cooperating with supply chain partners with similar goals. The introduction of innovative packaging solutions, such as unit-dose packaging, digital-enabled tracking systems, and the continual development of barrier properties to enhance drug stability, will play a significant role in shaping the future direction of the packaging space.
KEY TAKEAWAYS
-
By CountryIn 2024, Germany accounted for the largest share (21.8%) of the Europe pharmaceutical packaging market.
-
By TypeBy type, the pre-fillable syringes segment is expected to register the highest CAGR (17.1%).
-
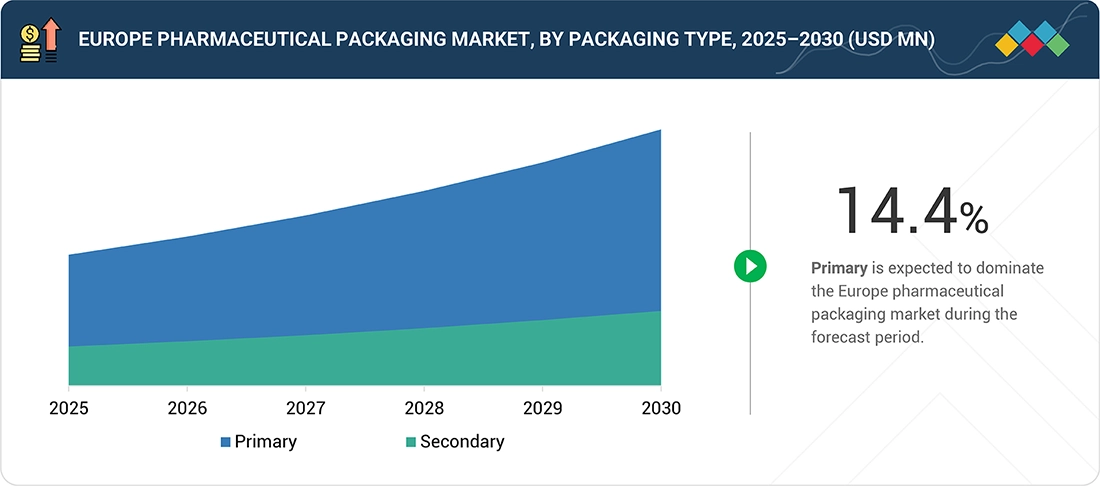
By Packaging TypeBy packaging type, the primary segment is expected to grow at the highest CAGR (14.4%).
-
By Drug DeliveryBy drug delivery, the injectables segment is expected to dominate the market.
-
Competitive Landscape - Key PlayersAmcor plc, Gerresheimer AG, and Schott AG were identified as Star players in the market, as they have focused on innovation, have broad industry coverage, and possess strong operational & financial strength.
-
Competitive Landscape - StartupsMedica Packaging, PHARMA PACKAGING SYSTEMS LIMITED, and Sepha Ltd have distinguished themselves among startups and SMEs due to their strong product portfolios and business strategies.
Europe Pharmaceutical Packaging this relates to the materials and processes used for the protection and delivery of pharmaceutical products to the oral, injectable, inhalation, and topical routes of administration. Pharmaceutical packaging plays a significant role in the stability and sterility of the medication as it offers protection against contamination and the delivery of the accurate dose of the medication throughout the entire shelf life of the product. Pharmaceutical packaging relates to the variety of available options, which may include plastic bottles, blister cards, vials, ampoules, prefilled syringes, tubes, pouches, and cartons, depending on the pharmaceutical formulation of the medication. The major functionalities of pharmaceutical packaging may include the protection of the medication from moisture, oxygen, and light, tamper evidence, convenience, and functionality with active pharmaceutical ingredients.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
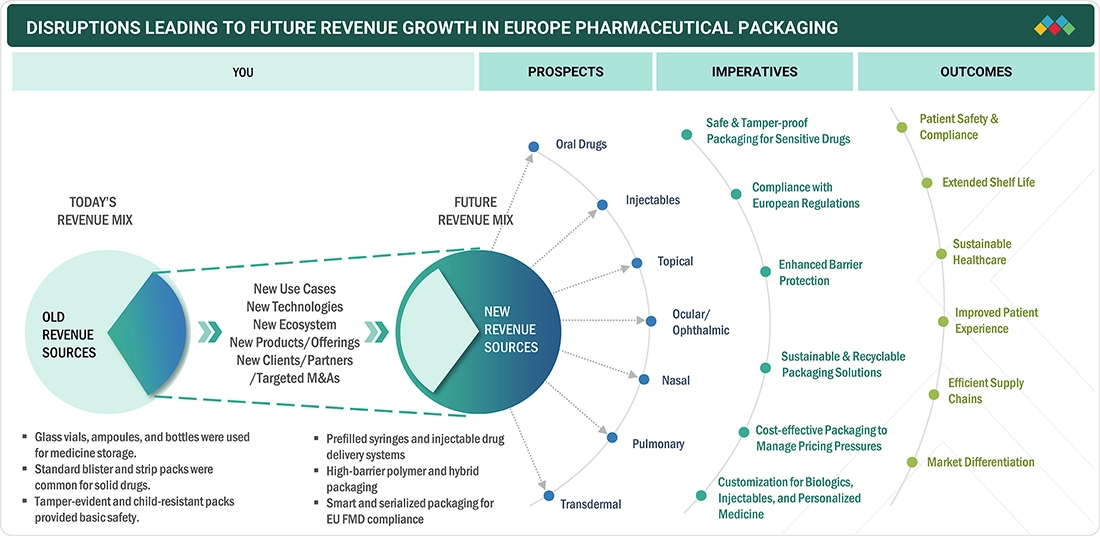
The Europe pharmaceutical packaging market faces a set of challenges and shifts in demands and regulatory and industry dynamics. Companies including pharmaceutical giants, Contract Development and Manufacturing Organizations, and Hospital and Retail Pharmacy Chains are the demand drivers for primary and secondary pharmaceutical packages, while the end-users are patients and professionals from the pharmaceutical industry. The industry is being driven by, or at least affected by, increasing demand for patient-centric packages (such as pre-filled syringes and unit-of-use packages), Green Deal sustainability and recyclability, Smart and Track-and-Trace packages, and EU GMP and Serialization regulations. Fluctuations in pharmaceutical production volumes, generic drug penetration, and healthcare spending across Europe directly affect packaging demand, shaping revenue stability and competitive positioning for European packaging suppliers.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Strong pharmaceutical and biotechnology manufacturing base in Europe

-
Rising demand for biologics, injectables, and advanced drug delivery systems
Level
-
High compliance and certification costs under EU GMP, FMD, and sustainability rules
-
Rising raw material and energy costs across European manufacturing
Level
-
Growing demand for sustainable and recyclable pharmaceutical packaging
-
Expansion of specialty drugs, biologics, and personalized medicine
Level
-
Managing complex EU serialization and traceability requirements
-
Balancing innovation, sustainability, and cost competitiveness
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Strong Pharmaceutical and Biotechnology Manufacturing Base
The European region boasts one of the most advanced pharma and biotech manufacturing bases in the world with leading pharmaceutical manufacturing, contract manufacturing organizations, and R&D facilities in Germany, France, Italy, Switzerland, and the UK. The well-developed manufacturing base ensures high demand for primary and secondary packaging such as vials, blister packages, cartridges, and sterilized packs, thereby ensuring high volumes of packaged pharma products in the market, either in the brand segment or generic segment.
Restraint: High Compliance and Certification Costs
European packaging companies in the pharmaceutical industry are required to adhere to strict regulations like EU GMP, Falsified Medicines Directive, and sustainability and recycling regulations. Implementation of these regulations entails high spending on the provision of cleanroom infrastructure and quality systems and traceability systems. Small to medium companies in the packaging industry face profitability and scalability challenges due to the high costs of complying with the regulations mentioned above.
Opportunity: Growing Demand for Sustainable and Recyclable Packaging
Sustainability goals under the EU Green Deal, along with the ESG commitments of pharmaceutical companies, are driving the switch towards recyclable, lightweight, and bio-based packaging. In such a way, the demand for the mentioned technologies and innovations, including mono-material blister packs, low-carbon glass, and paper-based secondary packaging, exists in the market. Those who are able to offer valid, compliant, and environmental packaging solutions can gain competitive advantage in the European pharmaceutical markets.
Challenge: Managing EU Serialization and Traceability Requirements
The EU Falsified Medicines Directive has developed codes that are unique, tamper-proof, and traced in real time for prescribing medicines.It is quite difficult for organizations to implement and maintain processes using different packaging lines. A simple mistake in implementation could lead to failure in supply chains, resulting in financial losses in terms of penalties and time for the delivery of drugs.
EUROPE PHARMACEUTICAL PACKAGING MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
A prominent brand of injectable pharmaceuticals who are one of the leaders in their market area collaborated with Gerresheimer AG to create COP/COC plastic special vials and cartridges for the treatment of biotherapies. | Provided the best product stability, delamination strength, break strength over standard glass, and compatibility for EMA and EU GMP directives for parenteral packages. |
 |
A leading European Pharma brand presented their oral solid dose products in Amcor high barrier blister packages and Cold Form Foil solutions that were compliant. | It provided high moisture and oxygen barrier protection and allowed for reliable serialization printing and optimization of film convertibility, besides meeting EU Falsified Medicines Directive (FMD) traceability. |
 |
An EU Injectable drug developer moved to the precision glass vials offering low-extractables formulation of the firm for sensitive biologic products and vaccines. | It delivered outstanding chemical inertness, improved sterility assurance, dimensional tolerances, and reproducible filling performance to meet EU regulatory expectations. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe pharmaceutical packaging ecosystem has been well-integrated, operating with the aim of ensuring the safety of drugs in this market. This would include suppliers of pharmaceutical-grade polymers, glass, aluminum, and paperboard, as well as integrators of packaging devices. They would all be working within the guidelines of the EU GMP/Falsified Medicines Directive. They are all highly connected within the pharmaceutical and biotech industry as a whole. The increasing demand for biologics, injectable drugs, and sustainable packaging, as well as the stringent standards of serialization, traceability, and recycling, has simply underscored the importance of a highly optimized value chain that stretches from material innovation to precise manufacturing and digital compliance solutions to guarantee safe and efficient drug distribution to patients in Europe.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Pharmaceutical Packaging Market, by Type
Prefilled syringes are expected to witness the fastest growth in the pharmaceutical packaging market in the forecast period. The demand for prefilled syringes is expected to be driven by their ease of use, accuracy, reduced drug waste, and safety features, compared to glass vials. The move toward using prefilled syringes has been fueled by trends in chronic disorders worldwide, the rising number of users seeking self-administration, as well as the swift adoption of biological and biosimilar drugs into the healthcare system. Pharmaceutical manufacturers and providers view prefilled syringes as preferable due to the lower risk of patient contamination, simplified adherence, and support of single-use sterile applications. To enhance patient safety, sterility, and the ultimate increase of global demand, regulators (e.g., FDA, EMA) are also advocating for packaging configurations. Recent innovations are continuing to promote their use in both developed and emerging healthcare markets with dual chamber designs for products, systems for needle safety, and advanced components that promote enhanced drug stability.
Europe Pharmaceutical packaging Market, by Packaging Type
The demand for primary packaging will be the highest in the Europe pharmaceutical packaging market, as it has applications in the safety, efficacy, and patient compliance aspects of medications. The types of primary packaging include blisters, bottles, prefilled syringes, and vials, all of which are highly important as protective packaging solutions for biological drugs that require resistance to contamination, moisture, and light while retaining stability. Due to the ever-growing trend towards biopharmaceuticals, injection-based medications, and personalized medications, there has been a rising demand for new packaging that is tamper-resistant and child-resistant. Finally, the anticipated investment levels in smart packaging will drive strong growth in primary packaging. Examples of this smart packaging will include serialization, QR codes, and real-time monitoring.
Europe Pharmaceutical Packaging Market, by Drug Delivery
In the Europe pharmaceutical packaging market, the drug-delivery segment for injectables is expected to dominate during the forecast period, driven by the region’s strong pipeline of biologics, vaccines, and specialty therapies. Injectable drug delivery products have seen extensive application of cutting-edge primary packaging like vials, prefilled syringes, cartridges, and auto-injectors that offer guarantees of sterility, accuracy of dosage, and stable temperature and light-sensitive drug products. The rising trends of monoclonal antibodies, biosimilars, oncology drugs, and chronic care injectables in Europe are boosting the demand for high-barrier glass and polymer syringes and integrated safety devices. At the same time, very strict regulations on EU GMP and Falsified Medicines Directive are fueling innovations in tamper-proof, trackable, and patient-safe packaging of injectables, followed by R&D spending on smart and connected injectors that expand the bright future of this market.
REGION
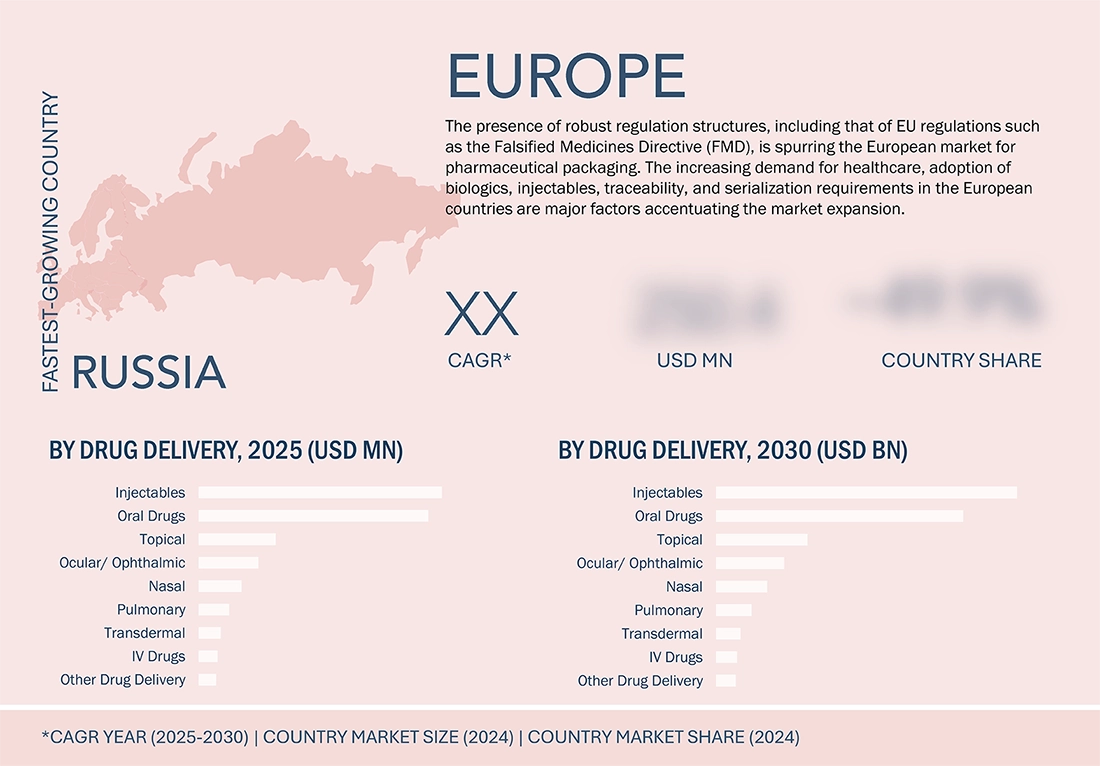
Russia to be the fastest-growing country in the Europe pharmaceutical packaging market during the forecast period
The Europe pharmaceutical packaging market is anticipated to experience constant and robust growth, and Russia is expected to be the foremost-growing country for the forecast period owing to the constant growth of the pharmaceutical manufacturing industry, increasing demand for health services, and rising concerns of the government towards pharmaceutical self-reliability. The pharmaceutical and biotechnology industry, and increasing production of generics, vaccines, and injectables, in the region, is driving the demand for superior quality primary and secondary packaging products. The trend in Russia is developing country-specific packaging and pharmaceutical filling capacities as part of the import substitution policies and the modernization of the health services of the country, accelerating the demand for pharmaceutical packaging products like vials, blister packaging, bottles, and appropriate labeling systems. The strict regulations of the EU, related to the serialization, anti-counterfeiting, and sustainability of pharmaceutical packaging, in the entire Europe region, is further fueling the pharmaceutical packaging market.
EUROPE PHARMACEUTICAL PACKAGING MARKET: COMPANY EVALUATION MATRIX
In the Europe pharmaceutical packaging market, Amcor plc (Star) has a commanding position based on its large geographical reach in Europe, diversified product offering that includes flexible, rigid, and specialty pharmaceutical packaging, and its leadership role in the development of sustainable & compliant pharmaceutical packaging solutions. The fact that Amcor is able to support large multi-national pharmaceutical manufacturers, in addition to smaller pharmaceutical manufacturers operating on the European market, while also pursuing the development of recyclable & low-carbon pharmaceutical packaging solutions, cements its position as a leader in the market. Gerresheimer AG (Emerging Leader) is methodically enhancing its current market position based on its intense focus on the development of primary pharmaceutical packaging for injectables, vials, syringes, and high-end drug delivery systems. Its geographic focus on the European market for manufacturing & innovative development, along with the market-driven trend for the increasing utilization of biologics, vaccines, & specialty pharmaceuticals, is rapidly pushing the company toward the leader position.
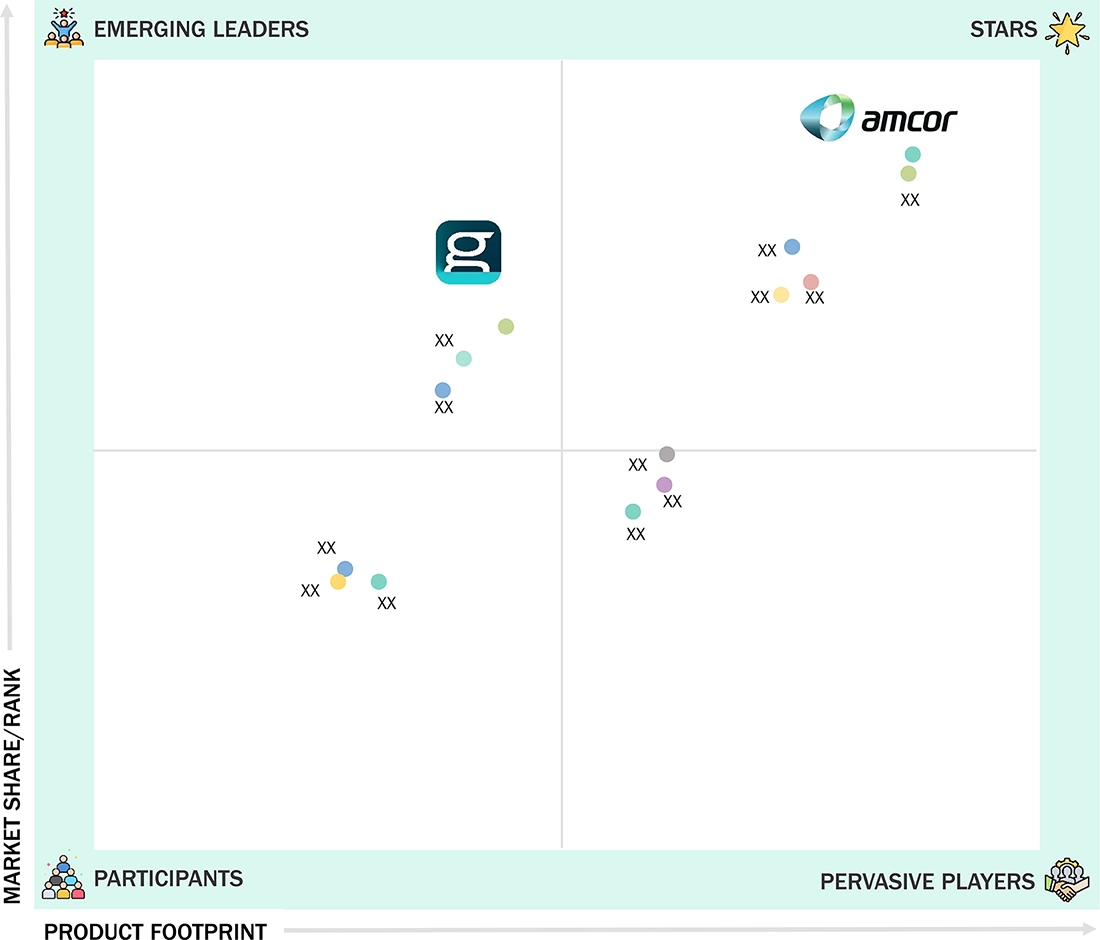
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Amcor plc (Switzerland)
- Gerresheimer AG (Germany)
- Schott AG (Germany)
- Nolato AB (Germany)
- Smurfit Westrock (Ireland)
- Pharma Packaging (UK)
- Stevanato Group (Italy)
- GAPLAST GmbH (Germany)
- SGD Pharma (France)
- Vetter Pharma (Germany)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 43.33 Billion |
| Market Forecast in 2030 (value) | USD 95.78 Billion |
| Growth Rate | CAGR of 14.2 % from 2025 to 2030 |
| Years Considered | 2022–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million), Volume (Kiloton) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, France, Italy, the UK, Spain, Russia, and the Rest of Europe. |
WHAT IS IN IT FOR YOU: EUROPE PHARMACEUTICAL PACKAGING MARKET REPORT CONTENT GUIDE
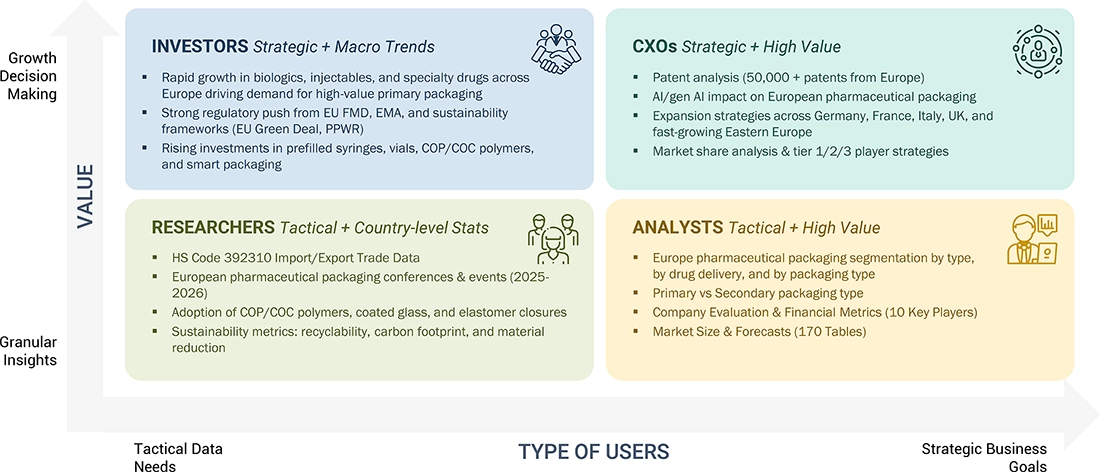
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| European Pharmaceutical Packaging Producer |
|
Identify high growth segments (injectables, biologics, and specialty drugs) and identify bottlenecks in serialization systems, compatibility between sterile fill and closing, sustainable materials. Target high-value pharmaceutical clients and CMOs. Monitoring of regulatory inflection points under EU GMP, FMD, and sustainability mandates. |
| Pharmaceutical & Biotech Company | The comparison of primary and secondary packaging solutions for different drug categories, shelf life, patient safety, and regulatory requirements. The mapping of qualified European packaging suppliers for injectables, oral drugs, and specialty therapies. Assess smart packaging, tamper evidence, and cold-chain compatibility. | Supplier qualification and faster packaging selection enabled. Improved packaging format for patient adherence and drug protection. Launch readiness services for biologics, vaccines, and special therapies in Europe provided. |
| Contract Manufacturing Organization (CMO) | The competitive analysis of European packaging partners for sterile fill-finish, labeling, blistering, and serialization. Mapping of integrated packaging and logistics capabilities. Analysis of sustainability-ready materials, traceability, and export and serialization regulations of EU countries. Outsourcing demand for packaging operations forecast. | Contract Manufacturing Organization (CMO). Competitive analysis of European packaging partners for sterile fill-finish, labeling, blistering, and serialization. Mapping of integrated packaging and logistics capabilities. Assessment of sustainability-ready materials, digital traceability, and compliance with EU export and serialization rules. Forecast of outsourcing demand for packaging operations. |
RECENT DEVELOPMENTS
- August 2025 : Schott AG inaugurated local production of FIOLAX glass tubing at its Jambusar, Gujarat facility (the first in India), addressing growing GLP-1 injectable demand with high-precision syringe and cartridge glass tubing.
- April 2025 : Amcor secured an order for AmSky, a recycle-ready, PVC-free thermoform blister system designed for healthcare and pharma, offering strong barrier performance and lower carbon footprint.
- January 2025 : Gerresheimer AG launched and marketed Gx Cap, a digitally networked closure enabling remote therapeutic monitoring and enhanced digital traceability for prescription medication containers.
Table of Contents

Methodology
The study involved four major activities to estimate the current size of the Europe pharmaceutical packaging market. Exhaustive secondary research was carried out to collect information on the market, the peer product market, and the parent product group market. The next step was to validate these findings, assumptions, and sizes with the industry experts across the value chain of Europe pharmaceutical packaging through primary research. The top-down and bottom-up approaches were employed to estimate the overall size of the Europe pharmaceutical packaging market. After that, market breakdown and data triangulation procedures were used to determine the size of different segments and sub-segments of the market.
Secondary Research
The market for the companies offering Europe pharmaceutical packaging is arrived at by secondary data available through paid and unpaid sources, analyzing the product portfolios of the major companies in the ecosystem, and rating the companies by their performance and quality. Various secondary sources, such as Business Standard, Bloomberg, World Bank, and Factiva, were referred to in order to identify and collect information for this study on the Europe pharmaceutical packaging market. In the secondary research process, various secondary sources were referred to identify and collect information related to the study. Secondary sources included annual reports, press releases, and investor presentations, forums, certified publications, and whitepapers. The secondary research was used to obtain critical information on the industry’s value chain, the total pool of key players, market classification, and segmentation from the market and technology-oriented perspectives.
Primary Research
In the primary research process, various primary sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side included industry experts, such as Chief Executive Officers (CEOs), Vice Presidents (VPs), marketing directors, technology and innovation directors, and related key executives from several key companies and organizations operating in the Europe pharmaceutical packaging market. After the complete market engineering (calculations for market statistics, market breakdown, market size estimations, market forecasting, and data triangulation), extensive primary research was conducted to gather information and verify and validate the critical numbers arrived at. Primary research was also conducted to identify the segmentation types, industry trends, competitive landscape of Europe pharmaceutical packaging offered by various market players, and key market dynamics, such as drivers, restraints, opportunities, challenges, industry trends, and key player strategies. In the complete market engineering process, the top-down and bottom-up approaches and several data triangulation methods were extensively used to perform the market estimation and market forecasting for the overall market segments and subsegments listed in this report. Extensive qualitative and quantitative analysis was performed on the complete market engineering process to list the key information/insights throughout the report.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The top-down and bottom-up approaches were used to estimate and validate the size of the Europe pharmaceutical packaging market. These approaches were also used extensively to estimate the size of various dependent market segments. The research methodology used to estimate the market size included the following:
Data Triangulation
After arriving at the overall market size using the market size estimation processes, the market was split into several segments and subsegments. The data triangulation procedure was employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Pharmaceutical packaging pertains to the materials and systems designed to protect, preserve, and deliver pharmaceutical products for oral, injectable, inhalation, and topical drug delivery modalities. It plays an important role in the stability and sterility of a drug, avoids contamination, and provides accurate dosing for the life of the product. Pharmaceutical packaging denotes the numerous varieties of available options, like plastic bottles, blister packs, vials, ampoules, prefillable syringes, tubes, pouches, and cartons to meet drug formulation requirements and regulatory obligations. Some of the significant functionalities of pharmaceutical packaging include moisture, oxygen, and light barrier protection, tamper evidence, patient convenience, and functionality with active pharmaceutical ingredients (APIs).
Areas of interest include ISO 15378 relating specifically to primary packaging materials, ISO 9001 regarding quality management systems, and GMP compliance. The DSCSA and the EU FMD identified serialized, tracking, and anti-counterfeiting programs, creating an opportunity for the packaging industry to help with drug quality and patient safety.
Stakeholders
- Europe pharmaceutical packaging manufacturers
- Raw material suppliers
- Converters & processors
- Distributors and traders
- Industry associations and regulatory bodies
- End users
Report Objectives
- To define, describe, and forecast the size of the Europe pharmaceutical packaging market, based on type, packaging type, drug delivery, raw material, and region in terms of value and volume
- To provide detailed information on the significant drivers, restraints, opportunities, and challenges influencing the market
- To strategically analyze micromarkets concerning individual growth trends, prospects, and their contribution to the market
- To assess the growth opportunities in the market for stakeholders and provide details on the competitive landscape for market leaders
- To strategically profile key players and comprehensively analyze their market shares and core competencies
- To analyze competitive developments such as acquisitions, product launches, expansions, partnerships, and agreements in the Europe pharmaceutical packaging market
- To provide the impact of AI/Gen AI on the market
Available Customizations
With the given market data, MarketsandMarkets offers customizations according to client-specific needs.
The following customization options are available for the Europe pharmaceutical packaging market report:
Product Analysis
- A product matrix that gives a detailed comparison of the product portfolio of each company
Geographic Analysis as per Feasibility
- A further breakdown of the Europe pharmaceutical packaging market for additional countries
Company Information
- Detailed analysis and profiling of additional market players (up to five)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Pharmaceutical Packaging Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Europe Pharmaceutical Packaging Market