
Medical Stick-to-Skin Adhesives Market Size, Growth, Share & Trends Analysis
Medical Stick-to-Skin Adhesives Market by Product (Acrylic, Silicone, Rubber), Backing Material, Type, Application (Surgery, Wound Care, Ostomy Seal), End User (Hospital, Clinic, Home Care), Key Stakeholder & Buying Criteria, Unmet Need - Forecast to 2030




OVERVIEW
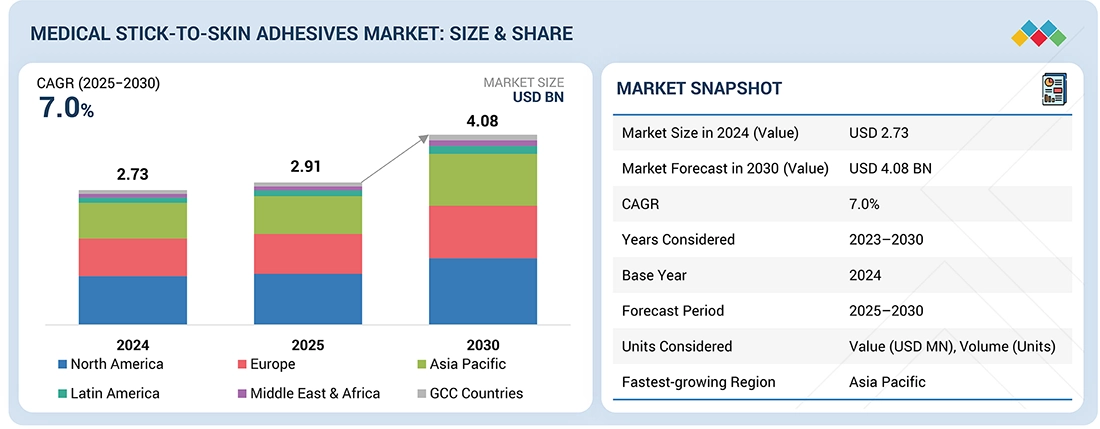
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The global medical stick-to-skin adhesives market, valued at US$2.74 billion in 2024, stood at US$2.91 billion in 2025 and is projected to advance at a resilient CAGR of 7.0% from 2024 to 2030, culminating in a forecasted valuation of US$4.08 billion by the end of the period. The medical stick-to-skin adhesives market is driven by rising surgical volumes, growing adoption of wearable medical devices, and increasing demand for gentle, extended-wear dressings for chronic wounds and aging populations. Advancements in silicone, acrylic, and hybrid adhesive technologies further accelerate adoption. However, pricing pressure from hospital tenders and private-label competition restrains market growth. Expanding home care usage, wearable health monitoring, and emerging-market adoption present strong opportunities for future growth.
KEY TAKEAWAYS
-
BY REGIONThe Asia Pacific is projected to witness the highest CAGR of 7.9% in the medical stick-to-skin adhesives market during the forecast period. Growth in this market is driven by the rapid expansion of healthcare infrastructure, rising surgical volumes, and the increasing adoption of advanced wound care and wearable medical devices across China, India, and Southeast Asia.
-
BY PRODUCTBy product, silicone-based adhesives are expected to register the highest CAGR of 8.1% during the forecast period. Growth in the market is driven by their superior gentleness, atraumatic removal, and excellent performance on fragile, sensitive, or aged skin, making them ideal for fast-growing segments such as advanced wound care, pediatrics, geriatrics, and wearable medical devices.
-
BY BACKING MATERIALBy backing material, other materials are projected to record the highest CAGR of 7.6% during the forecast period, as they enable advanced moisture management, conformability, and comfort demanded in modern wound care and wearable fixation.
-
BY END USERBy end user, home care settings are expected to register the highest CAGR of 7.4% in the medical stick-to-skin adhesives market during the forecast period. Growth in this market segment can be attributed to the increasing shift of chronic disease management away from hospitals toward self-care and the rising demand for easy-to-use adhesive dressings, device fixation patches, and long-wear products for diabetes, wound management, and elderly care.
-
COMPETITIVE LANDSCAPE - Key PlayersSolventum, Coloplast Group, Johnson & Johnson, Avery Dennison Corporation, and Smith & Nephew Plc were identified as Star players in the medical stick-to-skin adhesives market, as they have focused on innovation, broad industry coverage, and strong operational & financial strength.
-
COMPETITIVE LANDSCAPE - Start UpsParafix, Mactac, and Tapecon have distinguished themselves among startups and SMEs due to their strong product portfolio and business strategy.
The medical stick-to-skin adhesives market is driven by the rising adoption of wearable medical devices, the growing demand for advanced wound care dressings, and the increasing prevalence of chronic conditions requiring long-term skin fixation solutions. Technological advancements in silicone, acrylic, and hybrid adhesives enable extended wear, improved breathability, and enhanced tolerance for sensitive skin, further driving market growth. Expanding home-care use and a shift toward gentle-skin solutions in hospitals also fuel demand. A key restraint is pricing pressure from hospital tenders and private-label competition
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The medical stick-to-skin adhesives market is rapidly evolving, driven by advanced adhesive chemistries, biomaterials, and integration with digital wound care and wearable device ecosystems. Innovations—such as silicone gentleness for fragile skin, bioactive/antimicrobial formulations, extended-wear, breathable films, and bespoke die-cut components—are enhancing adhesion reliability, patient comfort, and infection control. Growth is boosted by rising ambulatory and home-care procedures, an aging population with fragile skin, and demand for faster workflows that reduce nursing time. As clinical evidence and device compatibility expand, adhesives enable better outcomes, lower total cost of care, and broader adoption across hospital and consumer channels.
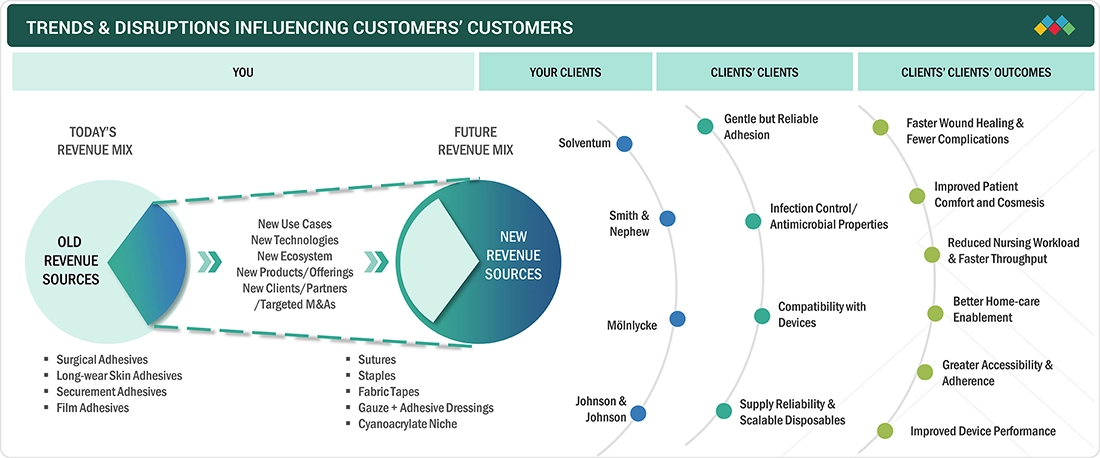
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing demand for minimally invasive surgical procedures

-
Advancements in wearable medical devices
Level
-
Regulatory compliance and safety concerns
Level
-
Expanding healthcare infrastructure in emerging economies
Level
-
Need to balance functionality with skin-friendliness
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Advancements in wearable medical devices
Advancements in wearable medical devices are significantly driving the medical stick-to-skin adhesives market. As continuous monitoring systems, smart patches, insulin pumps, and remote-care sensors become more sophisticated, the need for high-performance adhesives that ensure secure, long-term skin attachment has surged. Newer wearables require breathable, sweat-resistant, hypoallergenic, and flexible adhesives that maintain strong adhesion without irritating the skin. This growing dependency on reliable adhesive–skin interfaces is expanding demand, positioning advanced stick-to-skin adhesives as an essential enabler of next-generation wearable healthcare solutions.
Restraint: Regulatory compliance and safety concerns
Regulatory compliance and safety concerns act as key restraints in the medical stick-to-skin adhesives market. Adhesives must meet stringent FDA, CE, and ISO biocompatibility standards to ensure they do not cause irritation, sensitization, or long-term skin damage. Achieving approval requires extensive testing, documentation, and clinical validation, increasing development time and costs. Safety issues such as allergic reactions, poor adhesion, or residue can lead to product recalls or limited adoption. These regulatory complexities slow market entry and hinder smaller manufacturers from scaling quickly.
Opportunity: healthcare infrastructure in emerging economies
Expanding healthcare infrastructure in emerging economies presents a strong opportunity for the medical stick-to-skin adhesives market. Growing investments in hospitals, ambulatory centers, and home-care services are increasing demand for reliable wound-care and securement solutions. As countries improve surgical capacity, adopt modern infection-control standards, and strengthen chronic disease management, the need for advanced, skin-friendly adhesives rises sharply. Additionally, government-led health initiatives and rising affordability support wider adoption, creating significant potential for both premium and cost-effective adhesive products across fast-developing regions.
Challenge: Need to balance functionality with skin-friendliness
Balancing functionality with skin-friendliness remains a major challenge in the medical stick-to-skin adhesives market. Products must deliver strong, long-lasting adhesion to secure devices or dressings, yet remain gentle enough to avoid irritation, stripping, or allergic reactions—especially for pediatric, geriatric, and patients with sensitive skin. Achieving this balance requires complex formulation work involving breathable films, low-trauma chemistries, and controlled peel strength. However, enhancing gentleness can reduce adhesion reliability, while stronger adhesives may compromise comfort, making optimization difficult and costly for manufacturers.
Medical Stick-to-Skin Adhesives Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Broad portfolio of skin-compatible medical adhesives and film dressings for surgical incision closure, IV/tubing securement, transparent wound dressings, and wearable device attachment | Reliable adhesion with skin-friendly formulations | Reduces device failure and dressing changes | Improves clinician workflow and supports home care |
 |
Advanced wound care adhesives are integrated into dressings, negative-pressure systems, and ostomy/seal products, focusing on both chronic and acute wounds | Speeds wound management | Lowers infection risk and dressing-change frequency | Improves patient comfort and healing outcomes |
 |
Sterile adhesive dressings and securement systems for surgical sites, line fixation, and postoperative wound management with a focus on atraumatic removal | Minimizes skin trauma (esp. fragile/elderly skin) | Enhances cosmesis | Reduces nursing time and dressing-related complications |
 |
Surgical adhesive solutions and topical skin adhesives for incision closure as suture/staple alternatives, plus securement products for clinical settings | Enables faster OR closure, lower scarring, and fewer follow-up dressing changes | Potential reductions in procedure time and infection rates |
 |
High-performance flange/skin barriers and adhesives for ostomy appliances and peristomal skin protection | Products for long-wear home use | Improves seal reliability | Reduces leakage and skin irritation | Increases wearer confidence and adherence to ostomy care |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The medical stick-to-skin adhesives market operates through an interconnected ecosystem of raw-material suppliers (acrylate, silicone, hydrocolloid chemistries), specialty polymer and PSA manufacturers, and precision converters who produce tapes, films, and die-cut components. Adhesive formulators, wound-care firms, and device OEMs (Solventum, Smith & Nephew, Mölnlycke, Ethicon, B. Braun, Coloplast, and many startups) develop clinical and wearable solutions, supported by contract manufacturers with ISO-13485 capabilities. Products reach users via global medical distributors, hospital supply chains, and direct OEM channels. Key end users include hospitals, ambulatory surgical centers, home care providers, ostomy patients, and wearable device manufacturers focused on reliable adhesion, skin safety, and regulatory compliance.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
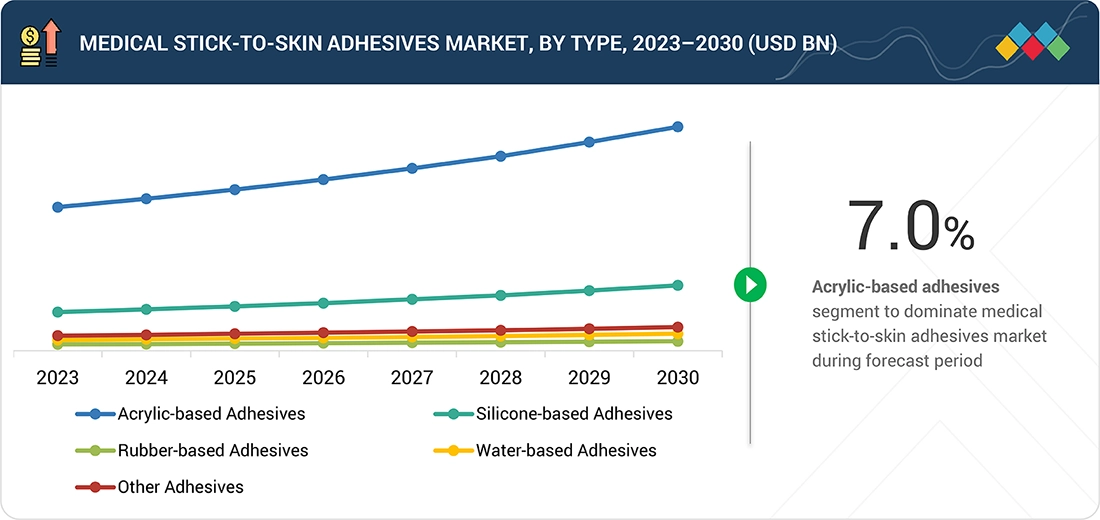
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Medical Stick-to-skin Adhesives Market, By Product
Silicone-based adhesives are expected to register the highest CAGR due to their superior gentleness, atraumatic removal, and excellent performance on fragile, sensitive, or aged skin, making them ideal for fast-growing segments such as advanced wound care, pediatrics, geriatrics, and wearable medical devices. Their breathability, extended-wear comfort, and low-residue properties drive strong clinician and OEM preference, accelerating the adoption of premium products globally.
Medical Stick-to-skin Adhesives Market, By Backing Material
Other materials are projected to record the highest CAGR, as they enable advanced moisture management, conformability, and comfort demanded in modern wound care and wearable fixation. Their ability to support extended wear, protect fragile skin, and integrate with next-generation adhesive chemistries makes them the preferred choice for premium dressings and specialized clinical applications.
Medical Stick-to-skin Adhesives Market, By End User
Home care settings are expected to post the highest CAGR in the medical stick-to-skin adhesives market as chronic disease management shifts away from hospitals toward self-care, driving demand for easy-to-use adhesive dressings, device fixation patches, and long-wear products for diabetes, wound management, and elderly care. Rising adoption of remote monitoring devices, CGMs, and home wound-care programs further accelerates the need for reliable, gentle stick-to-skin adhesives.
REGION
Asia Pacific to be fastest-growing region in global medical stick-to-skin adhesives market during forecast period
The APAC is projected to witness the highest CAGR in the medical stick-to-skin adhesives market due to the rapid expansion of healthcare infrastructure, rising surgical volumes, and increasing adoption of advanced wound-care and wearable medical devices across China, India, and Southeast Asia. Growing diabetic and elderly populations further drive demand for long-wear dressings and device fixation products. Additionally, strong local manufacturing, lower production costs, and increasing investments by global players accelerate market penetration. Government initiatives improving healthcare access also support faster regional growth.
Medical Stick-to-Skin Adhesives Market: COMPANY EVALUATION MATRIX
In the medical stick-to-skin adhesives market matrix, Solventum (Star) leads with a dominant global footprint, an extensive portfolio spanning surgical adhesives, securement tapes, film dressings, and medical-grade silicones, as well as deep distribution into hospitals and device OEM channels. Its scale, formulation expertise, and integrated supply-chain capabilities—coupled with robust clinical evidence and OEM partnerships—cement leadership. Mölnlycke (Emerging Leader) is gaining momentum by focusing on atraumatic adhesives, advanced wound-care dressings, and solutions for fragile skin, positioning itself for accelerated growth in acute and home-care segments. While 3M retains clear market leadership, Mölnlycke’s targeted product differentiation, strong wound-care positioning, and geographic expansion show potential to move toward the leaders’ quadrant as it broadens offerings and market reach.
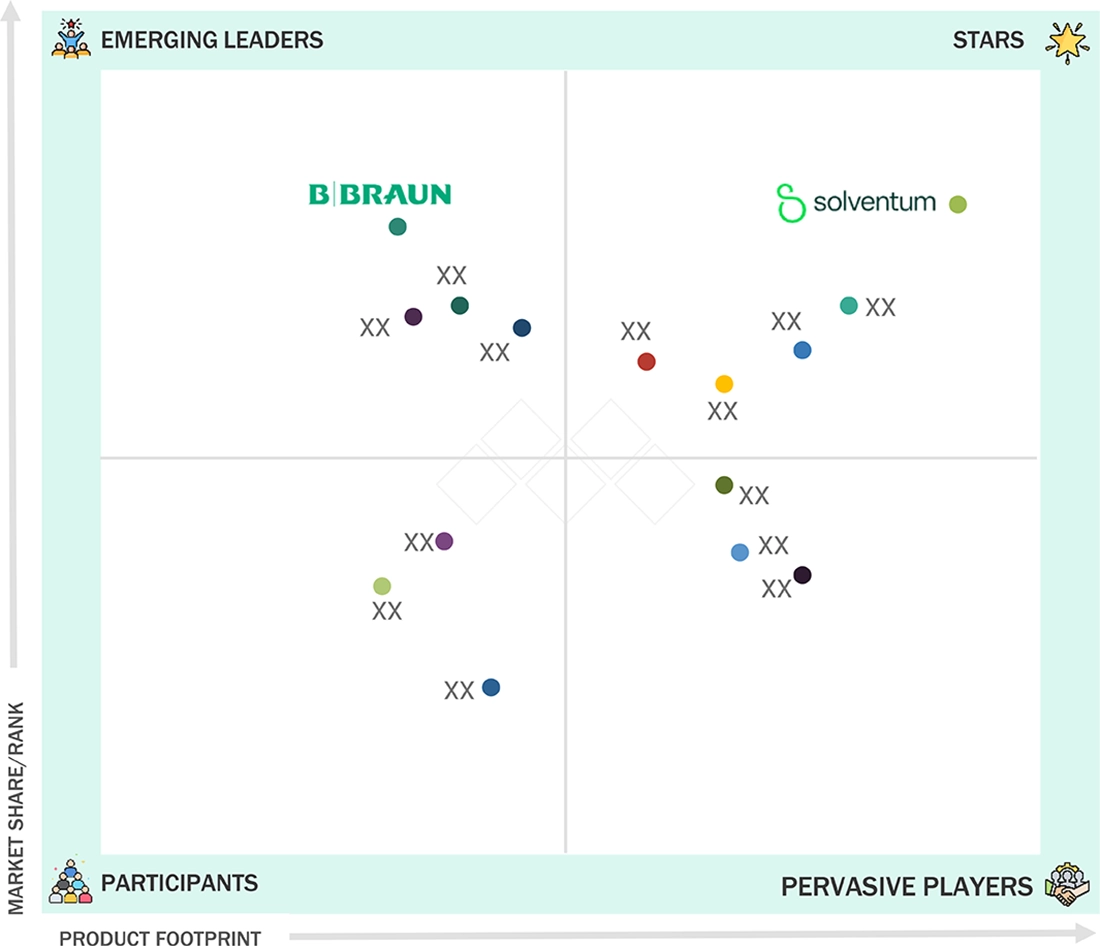
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Solventum (US)
- Johnson & Johnson (US)
- Coloplast Group (Denmark)
- Nitto Denko Corporation (Japan)
- Avery Dennison Corporation (US)
- Smith & Nephew PLC (UK)
- Henkel AG & Co. KGaA (Germany)
- DuPont (US)
- Mölnlycke Health Care AB (Sweden)
- Advanced Medical Solutions Group plc (UK)
- Nichiban Co., Ltd. (Japan)
- B. Braun Melsungen AG (Germany)
- HB Fuller (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.73 BN |
| Market Forecast in 2030 (Value) | USD 4.08 BN |
| Growth Rate | 7.0% |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million), Volume (Units) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered | By Product, Backing Material, Type, Application, End User, and Region |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, the Middle East & Africa, and GCC Countries |
WHAT IS IN IT FOR YOU: Medical Stick-to-Skin Adhesives Market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis |
|
|
| Company Information |
|
|
| Geographic Analysis |
|
|
RECENT DEVELOPMENTS
- September 2024 : Solventum launched the V.A.C. Peel and Place Dressing. This innovative, all-in-one dressing and drape design streamlined the application process, significantly reducing the time, effort, and training typically required for dressing changes. It includes a built-in perforated, non-adherent layer that helps prevent tissue ingrowth, allowing for gentler, less painful removal
- Jan-24 : Smith+Nephew announced plans to build a new R&D and manufacturing facility for its Advanced Wound Management franchise on the outskirts of Hull, UK. The new facility is expected to support more than USD 10 billion in sales during its first ten years of operation.
- June 2022 : Coloplast Group launched Biatain Silicone Fitin US. This silicone foam dressing with 3DFit Technology supports pressure injury prevention and wound management by fitting body contours and wound beds up to 2 cm deep, enhancing patient comfort and care.
Table of Contents

- 4.1 INTRODUCTION
-
4.2 MARKET DYNAMICSDRIVERSRESTRAINTSOPPORTUNITIESACHALLENGES
- 4.3 UNMET NEEDS AND WHITE SPACES
- 4.4 INTERCONNECTED MARKETS AND CROSS-SECTOR OPPORTUNITIES
- 4.5 STRATEGIC MOVES BY TIER-1/2/3 PLAYERS
- 4.6 REIMBURSEMENT ANALYSIS
- 5.1 PORTER’S FIVE FORCES ANALYSIS
-
5.2 MACROECONOMIC OUTLOOKINTRODUCTIONGDP TRENDS AND FORECASTTRENDS IN THE GLOBAL ADHESIVES INDUSTRY
- 5.3 SUPPLY CHAIN ANALYSIS
- 5.4 VALUE CHAIN ANALYSIS
- 5.5 ECOSYSTEM ANALYSIS
-
5.6 PRICING ANALYSISAVERAGE SELLING PRICE TREND, BY PRODUCT (2023-2025)AVERAGE SELLING PRICE TREND, BY REGION (2023-2025)
- 5.7 TRADE ANALYSIS
- 5.8 KEY CONFERENCES AND EVENTS, 2025–2026
- 5.9 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.10 INVESTMENT AND FUNDING SCENARIO
- 5.11 CASE STUDY ANALYSIS
-
5.12 IMPACT OF 2025 US TARIFF – MEDICAL STICK-TO-SKIN ADHESIVES MARKETINTRODUCTIONKEY TARIFF RATESPRICE IMPACT ANALYSISIMPACT ON COUNTRIES/REGIONS- US- EUROPE- APACIMPACT ON END-USE INDUSTRIES
- 6.1 KEY EMERGING TECHNOLOGIES
- 6.2 COMPLEMENTARY TECHNOLOGIES
- 6.3 TECHNOLOGY/PRODUCT ROADMAP
- 6.4 PATENT ANALYSIS
- 6.5 FUTURE APPLICATIONS
-
6.6 IMPACT OF AI/GEN AI ON MEDICAL STICK-TO-SKIN ADHESIVES MARKETTOP USE CASES AND MARKET POTENTIALBEST PRACTICES IN MEDICAL STICK-TO-SKIN ADHESIVES PROCESSINGCASE STUDIES OF AI IMPLEMENTATION IN THE MEDICAL STICK-TO-SKIN ADHESIVES MARKETINTERCONNECTED ADJACENT ECOSYSTEM AND IMPACT ON MARKET PLAYERSCLIENTS’ READINESS TO ADOPT GENERATIVE AI IN MEDICAL STICK-TO-SKIN ADHESIVES MARKET
- 8.1 DECISION-MAKING PROCESS
- 8.2 BUYER STAKEHOLDERS AND BUYING EVALUATION CRITERIA
- 8.3 ADOPTION BARRIERS & INTERNAL CHALLENGES
- 8.4 UNMET NEEDS FROM VARIOUS END-USE INDUSTRIES
- 9.1 INTRODUCTION
- 9.2 ACRYLIC-BASED
- 9.3 SILICONE-BASED
- 9.4 RUBBER-BASED
- 9.5 WATER-BASED
- 9.6 OTHERS (HYDROCOLLOID-BASED ADHESIVES, HYDROGEL-BASED ADHESIVES, POLYURETHANE-BASED ADHESIVES AND POLYMER-BASED ADHESIVES)
- 10.1 INTRODUCTION
- 10.2 PAPER
- 10.3 FABRIC
- 10.4 PLASTIC
- 10.5 OTHER MATERIALS (FOAM MATERIALS, HYDROCOLLOID MATERIALS, NONWOVEN MATDERIALS, POLYURETHANE FILMS, HYDROGEL MATERIALS AND SILICONE-COATED RELEASE LINERS)
- 11.1 INTRODUCTION
- 11.2 ELECTRODE ADHESIVES
- 11.3 TRANSDERMAL DRUG DELIVERY ADHESIVES
- 11.4 SPECIALIZED ADHESIVES
- 12.1 INTRODUCTION
- 12.2 SURGERY
- 12.3 WOUND DRESSING
- 12.4 OSTOMY SEALS
- 12.5 OTHER APPLICATIONS (RESPIRATORY CARE, IV AND CATHETER SECUREMENT, ORTHOPEDIC DEVICES, DERMATOLOGY TREATMENTS AND DIAGNOSTIC DEVICES.)
- 13.1 INTRODUCTION
- 13.2 HOSPITALS & CLINICS
- 13.3 HOME CARE SETTINGS
- 13.4 OTHER END USERS (EMERGENCY MEDICAL SERVICES, SPECIALTY HEALTHCARE PROVIDERS, SPORTS MEDICINE, AND ACADEMIC AND RESEARCH INSTITUTIONS)
- 14.1 INTRODUCTION
-
14.2 ASIA PACIFICCHINAJAPANINDIASOUTH KOREAAUSTRALIAREST OF ASIA PACIFIC
-
14.3 NORTH AMERICAUSCANADA
-
14.4 EUROPEGERMANYFRANCEUKITALYSPAINREST OF EUROPE
-
14.5 LATIN AMERICABRAZILMEXICOREST OF LATIN AMERICA
- 14.6 MIDDLE EAST & AFRICA
- 14.7 GCC COUNTRIES
- 15.1 OVERVIEW
- 15.2 KEY PLAYER COMPETITIVE STRATEGIES/RIGHT TO WIN
- 15.3 REVENUE ANALYSIS
-
15.4 MARKET SHARE ANALYSISGLOBAL MEDICAL STICK-TO-SKIN ADHESIVES MARKET SHARE ANALYSIS, 2024US MEDICAL STICK-TO-SKIN ADHESIVES MARKET SHARE ANALYSIS, 2024
- 15.5 BRAND COMPARISON
-
15.6 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024STARSEMERGING LEADERSPERVASIVE PLAYERSPARTICIPANTSCOMPANY FOOTPRINT: KEY PLAYERS, 2024- Company footprint- Region footprint- Product footprint- Backing Material footprint- Type industry footprint- Application industry footprint- End User industry footprint
-
15.7 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024PROGRESSIVE COMPANIESRESPONSIVE COMPANIESDYNAMIC COMPANIESSTARTING BLOCKSCOMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024- Detailed list of key startups/SMEs- Competitive benchmarking of key startups/SMEs
- 15.8 COMPANY VALUATION AND FINANCIAL METRICS
-
15.9 COMPETITIVE SCENARIOPRODUCT LAUNCHESDEALSEXPANSIONS
-
16.1 KEY PLAYERS3MSCAPA HEALTHCAREAVERY DENNISON MEDICAL
-
17.1 OTHER PLAYERSMACTACSEKISUI KASEI CO. LTD.SHURTAPE TECHNOLOGIES, LLCTESA SEPAUL HARTMANN
-
18.1 RESEARCH DATASECONDARY DATA- Key data from secondary sourcesPRIMARY DATA- Key data from primary sources- Key primary participants- Breakdown of primary interviews- Key industry insights
-
18.2 MARKET SIZE ESTIMATIONBOTTOM-UP APPROACHTOP-DOWN APPROACH
-
18.3 MARKET FORECAST APPROACHSUPPLY SIDEDEMAND SIDE
- 18.4 DATA TRIANGULATION
- 18.5 FACTOR ANALYSIS
- 18.6 RESEARCH ASSUMPTIONS
- 18.7 RESEARCH LIMITATIONS AND RISK ASSESSMENT
- 19.1 DISCUSSION GUIDE
- 19.2 KNOWLEDGESTORE: MARKETSANDMARKETS’ SUBSCRIPTION PORTAL
- 19.3 CUSTOMIZATION OPTIONS
- 19.4 RELATED REPORTS
- 19.5 AUTHOR DETAILS
Methodology
The study involved major activities in estimating the current size of the medical stick-to-skin adhesives market. Exhaustive secondary research was done to collect information on the medical stick-to-skin adhesives market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain using primary research. Different approaches, including top-down and bottom-up methods, were employed to estimate the total market size. After that, the market breakup and data triangulation procedures were employed to estimate the market size of the segments and subsegments within the medical stick-to-skin adhesives market.
Secondary Research
This research study involved the extensive use of secondary sources, including directories, databases such as Dun & Bradstreet, Bloomberg Businessweek, and Factiva, as well as whitepapers and company house documents. Secondary research was undertaken to identify and collect information for this extensive, technical, market-oriented, and commercial study of the medical stick-to-skin adhesives market. It was also used to gather important information about top players, market classification, and segmentation based on industry trends, as well as geographic markets and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various supply- and demand-side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, VPs/managing directors, marketing heads/directors and sales directors, marketing/sales managers, regional/area sales managers, export/import heads/managers, and other adhesive market-related personnel from various companies and organizations operating in the medical stick-to-skin adhesives market. Primary sources from the demand side included the vice presidents of hospitals and clinics, C-level executives, department heads, doctors/eye physicians, supply management teams of hospitals, clinic personnel, and laboratory technicians.
Breakdown of Primary Interviews
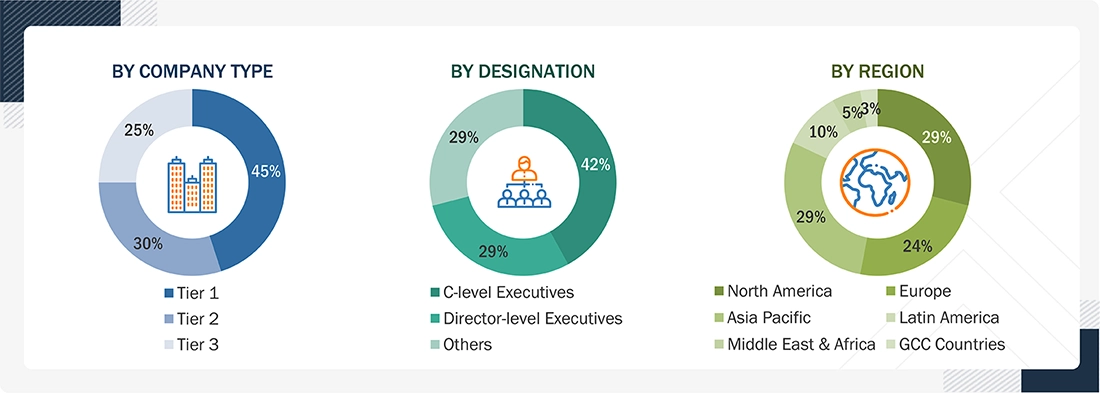
Note 1: C-level executives include CEOs, COOs, and CTOs.
Note 2: Others include sales, marketing, and product managers.
Note 3: Companies are classified into tiers based on their total revenue. As of 2024: Tier 1 = >USD 1 billion, Tier 2 = USD 500 million to USD 1 billion, Tier 3 = < USD 500 million
To know about the assumptions considered for the study, download the pdf brochure
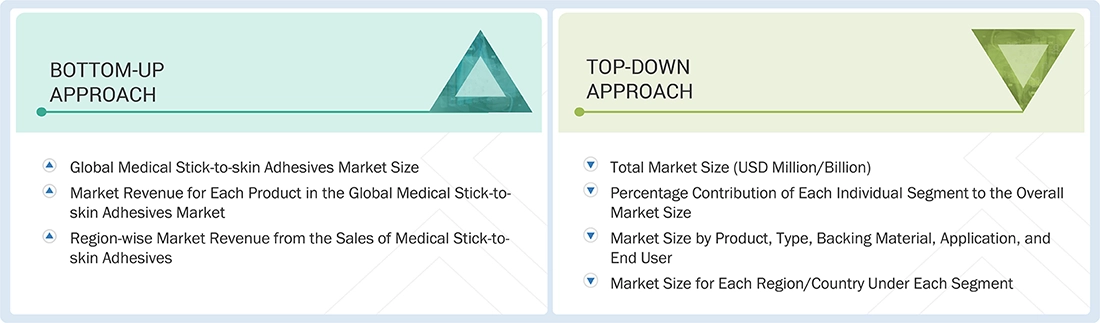
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the medical stick-to-skin adhesives market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
The entire market was split into four segments when the market size was determined. Data triangulation and market breakdown processes were used where necessary to complete the entire market engineering process and arrive at precise statistics for all segments.
Approach to derive the market size and estimate market growth.
Using secondary data from paid and unpaid sources, the market rankings for the major players were determined following a thorough analysis of their sales of medical stick-to-skin adhesives. Due to data restrictions, the revenue share in certain cases was determined after a thorough analysis of the product portfolio of big corporations and their individual sales performance. This information was verified at each stage by in-depth interviews with professionals in the field.
Market Definition
Medical stick-to-skin adhesives are specialized materials designed to bond securely with the skin while remaining gentle and hypoallergenic. These adhesives are used to affix medical devices, such as bandages, wound dressings, and monitoring equipment, ensuring they stay in place for effective treatment without causing skin irritation or discomfort.
Stakeholders
- Raw material suppliers
- Medical stick-to-skin adhesive manufacturers and distributors
- Manufacturing technology providers
- Industry associations
- Traders, distributors, and suppliers of medical stick-to-skin adhesives
- Government associations, bodies, and regional organizations
- Healthcare providers
- Regulatory bodies
- Healthcare professionals
- Research & development (R&D) organizations
- Academic institutions
- Quality control and testing laboratories
- Government health departments
- Environmental agencies
- Patients and patient advocacy groups
- Investors and financial institutions
Report Objectives
- To describe, analyze, and forecast the medical stick-to-skin adhesives market by product, backing material, application, end user, and region
- To describe and forecast the medical stick-to-skin adhesives market for key regions: North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and the GCC Countries
- To provide detailed information regarding drivers, restraints, opportunities, and challenges influencing the growth of the medical stick-to-skin adhesives market
- To strategically analyze the ecosystem, regulations, patenting trend, value chain, Porter’s five forces, and prices pertaining to the market under study
- To strategically analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for market players
- To profile key players and comprehensively analyze their market shares and core competencies in the medical stick-to-skin adhesives market
- To analyze competitive developments such as collaborations, acquisitions, product launches, expansions, and R&D activities in the medical stick-to-skin adhesives market
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Medical Stick-to-Skin Adhesives Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Medical Stick-to-Skin Adhesives Market