Pharmaceutical Manufacturing Execution System (MES) Market
Pharmaceutical Manufacturing Execution System (MES) Market By Offering (Software, Services), Deployment (On-premises, Cloud, Hybrid), Application (Production Management, Quality Management, Performance Analytics, Predictive Maintenance) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The Pharmaceutical Manufacturing Execution System (PMES) market is projected to reach USD 4.67 billion by 2030, up from USD 2.37 billion in 2025, at a CAGR of 14.3% from 2025 to 2030. Stringent regulatory compliance, rising biologics and vaccine production, demand for electronic batch records, and accelerated digitalization across pharma plants are key drivers boosting the growth of the pharmaceutical MES industry .
KEY TAKEAWAYS
-
BY RegionBy Region, Asia Pacific is expected to grow fastest at a CAGR of 16.0% during the forecast period.
-
BY OFFERINGBy Offering, the services segment is expected to register the highest CAGR of 15.3% during the forecast period.
-
BY DEPLOYMENTBy Deployment, the cloud-based segment is expected to dominate the market in terms of market share.
-
COMPETITIVE LANDSCAPE - KEY PLAYERSSiemens (Germany) and Dassault Systèmes (France) were identified as some of the star players in the Asia Pacific MES market, given their substantial market share and product footprint.
-
COMPETITIVE LANDSCAPE - STARTUPS/SMESEpicor Software Corporation (US) and Infor (US), among others, have distinguished themselves among SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
Stringent global regulations, the need for electronic batch records, and increasing focus on data integrity are pushing pharma manufacturers to adopt MES. Rapid growth in biologics, vaccines, and high-complexity drugs further drives digitalization, real-time monitoring, and automated quality control, strengthening MES adoption across global pharmaceutical facilities.
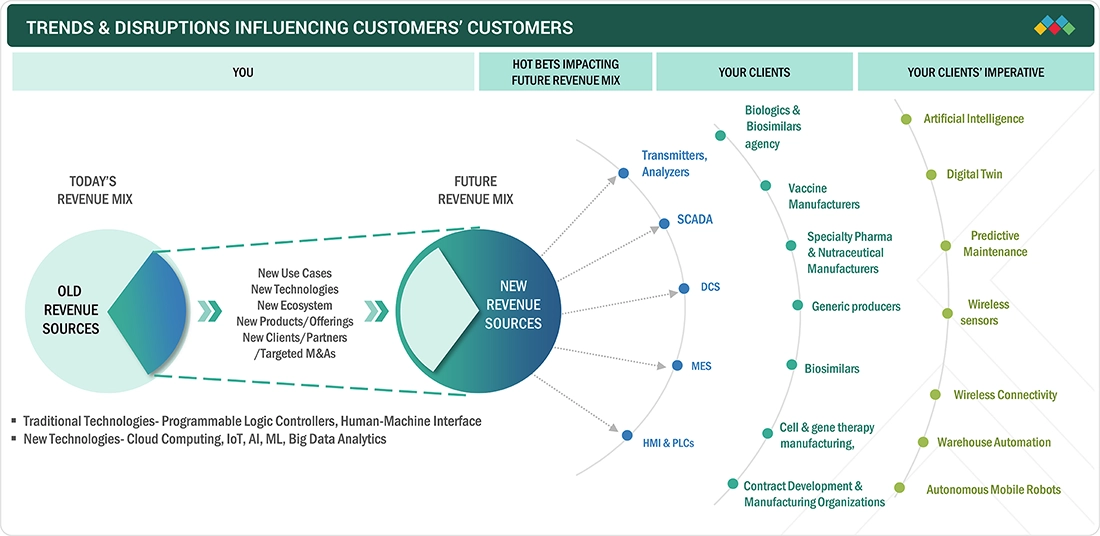
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The pharmaceutical MES market is witnessing major trends and disruptions driven by the rapid adoption of cloud-based and hybrid MES architectures, the increasing integration of AI for predictive quality and automated batch review, and a rising demand for paperless EBR systems. Advanced analytics, IoT-enabled equipment connectivity, and digital twins are transforming production visibility and decision-making. Additionally, the growth of biologics, cell & gene therapies, and adaptive manufacturing is accelerating modular, scalable MES deployments, disrupting traditional rigid systems and enabling more agile, compliant pharmaceutical operations
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Stringent global regulatory requirements

-
Rapid growth of biologics, vaccines, and high-complexity drug manufacturing
Level
-
High implementation and integration costs
-
Complex system validation and long deployment cycles
Level
-
Expansion of CDMOs and multi-site pharma networks
-
Integration of AI, IIoT, and advanced analytics
Level
-
Interoperability issues with existing ERP, LIMS, SCADA, and automation systems
-
Skill gaps and limited digital maturity
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Stringent Global Regulatory Requirements
Stringent regulatory frameworks such as US FDA 21 CFR Part 11, EU GMP Annex 11, PIC/S guidelines, and global data-integrity mandates are significantly accelerating MES adoption in the pharmaceutical sector. Manufacturers must ensure complete traceability, audit readiness, electronic signatures, and error-free batch documentation to comply with these regulations. MES enables electronic batch records, automated deviation handling, and real-time quality checks, reducing compliance risks. As regulatory scrutiny intensifies worldwide, pharmaceutical companies increasingly rely on MES to maintain consistent, validated, and compliant manufacturing operations.
Restraint: High Implementation and Integration Costs
Pharmaceutical MES deployments require a high upfront investment due to the complexity of system validation, customization, and integration with legacy automation, ERP, and quality systems. Older brownfield plants require substantial upgrades to support MES, driving up costs further. Additionally, long implementation timelines, extensive training, and stringent qualification (IQ/OQ/PQ) requirements add to the financial burden. For mid-sized and regional pharma companies, these costs often slow adoption, making it challenging to justify MES deployment despite its strong long-term operational and compliance benefits.
Opportunity: Expansion of CDMOs and Multi-Site Pharma Networks
The rapid growth of CDMOs and global multi-site pharmaceutical networks presents a significant opportunity for MES vendors. These organizations require standardized, scalable, and interoperable MES solutions to manage diverse client portfolios, complex formulations, and high-volume batch production. MES helps harmonize workflows, enable paperless manufacturing, and support efficient tech transfers across sites. As CDMOs expand biologics, vaccines, specialty drugs, and sterile operations, demand for cloud-enabled, multi-plant MES architectures is rising, creating substantial growth potential in the pharmaceutical MES market.
Challenge: Interoperability with ERP, LIMS, SCADA, and Automation Systems
Achieving seamless interoperability between MES and existing ERP, LIMS, SCADA, and automation systems remains a significant challenge in the pharmaceutical manufacturing industry. Many plants operate with heterogeneous, aging systems that lack standardized interfaces, resulting in inconsistent data exchange and costly integration. Poor interoperability can hinder real-time visibility, compromise data integrity, and delay the release of batches. Ensuring accurate synchronization of master data, equipment records, quality parameters, and process values requires significant engineering effort. This challenge slows MES deployments and creates operational bottlenecks in regulated pharma environments
pharmaceutical-manufacturing-execution-system-mes-market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Siemens Opcenter Execution Pharma supports end-to-end pharma manufacturing, including batch execution, EBR, weigh & dispense, serialization, and quality workflows. It integrates with DCS/SCADA, LIMS, and ERP to support GMP-compliant operations across sterile injectables, biologics, API, and solid-dose plants. | Enhances batch consistency, reduces deviations, and accelerates the electronic batch release process. Strengthens GMP compliance and data integrity. Provides real-time process visibility and harmonized multi-site operations for large pharma networks. |
 |
DELMIA Apriso Pharma MES is deployed for digital batch execution, quality management, shop-floor orchestration, and traceability in biologics, specialty pharma, and regulated formulations. It integrates tightly with 3DEXPERIENCE for unified PLM-MES-QMS operations.. | Enables faster tech transfers, improved batch genealogy, and higher right-first-time ratios. Harmonizes production workflows and supports rapid scale-up for new molecules. Ensures end-to-end traceability and regulatory audit readiness |
 |
SAP Digital Manufacturing for Pharma offers EBR, recipe execution, material traceability, and integrated quality review across global GMP facilities. It connects MES with SAP S/4HANA, maintaining unified master data and compliant workflows across API, formulations, and packaging units. | Improves global standardization, reduces manual documentation, and enhances audit trails. Accelerates batch release through real-time data flow. Strengthens compliance by integrating QMS, ERP, and MES for entirely paperless production |
 |
Yokogawa’s Pharma MES and automation suite supports batch control, weighing and dispensing, recipe execution, cleaning validation, and real-time process monitoring for highly sensitive biologics and sterile manufacturing lines. It integrates seamlessly with distributed control systems for continuous production reliability. | Reduces batch cycle variability, enhances precision in sterile/biologics workflows, and improves production throughput. Provides strong process stability and regulatory compliance. Ideal for high-purity, high-risk pharma environments. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The pharmaceutical MES ecosystem comprises MES software vendors, automation providers, cloud platforms, and system integrators, enabling end-to-end digital manufacturing. Collaboration among equipment suppliers, IT/OT integrators, and industry-specific solution partners supports rapid smart-factory deployment. Strong government programs, rising digital investments, and expanding manufacturing clusters further strengthen the region’s MES ecosystem.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Pharmaceutical Manufacturing Execution System Market, By Offering
Based on the offering, software contributes a significant share to the pharmaceutical MES market, as manufacturers increasingly prioritize digital platforms to streamline complex production workflows and ensure regulatory compliance. MES software enables real-time monitoring, electronic batch records, deviation management, and improved traceability—critical needs for pharma companies facing stringent global quality standards. Its ability to integrate with ERP, LIMS, and automation systems further enhances operational efficiency and data integrity. With rising adoption of smart manufacturing, paperless operations, and AI-driven analytics, software remains the core investment area, driving the largest share of spending within pharmaceutical MES implementations.
Pharmaceutical Manufacturing Execution System Market, By Deployment
Based on deployment, on-premises deployment contributes a significant share in the pharmaceutical MES market due to the industry’s heightened need for data security, control, and regulatory compliance. Pharmaceutical manufacturers prefer on-premises MES solutions because they allow for full ownership of sensitive production, batch, and quality data, while ensuring adherence to stringent GMP and FDA guidelines. These deployments provide high system reliability, flexibility in customization, and seamless integration with existing automation infrastructures. In environments where uptime, validation, and audit readiness are critical, on-premises MES platforms continue to dominate, especially among large pharmaceutical and biologics facilities with complex operational requirements.
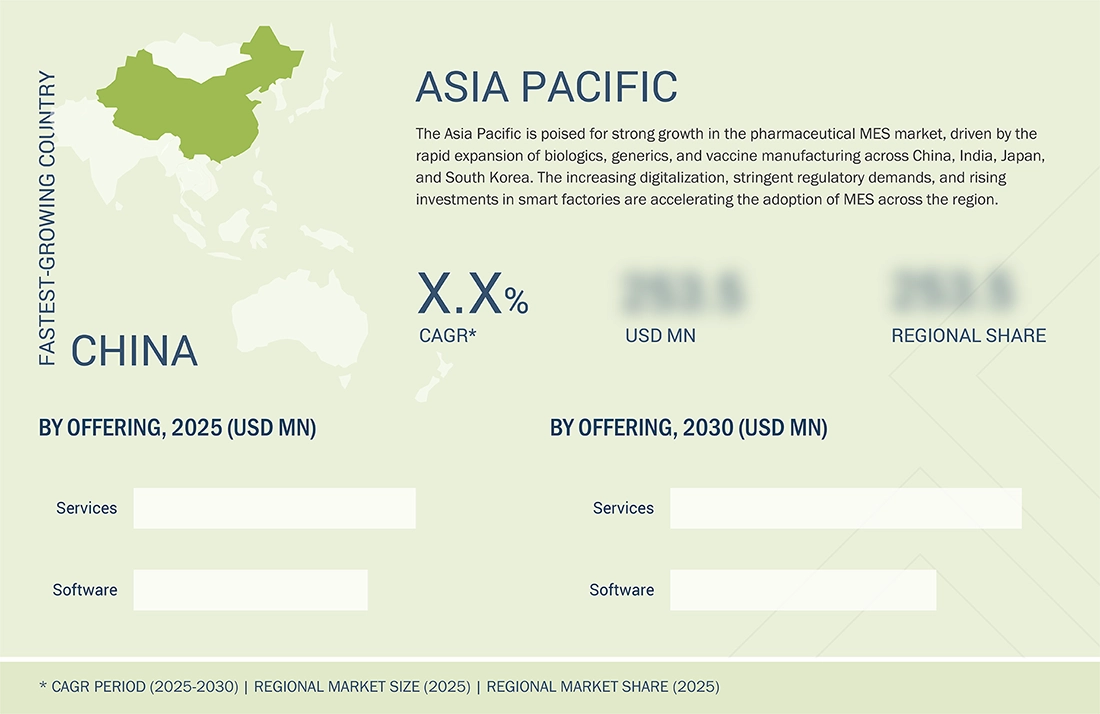
Pharmaceutical Manufacturing Execution System Market, By Asia Pacific
The Asia Pacific pharmaceutical MES market is driven by the rapid expansion of biologics, generics, and vaccine manufacturing, particularly in China, India, South Korea, and Japan. Growing regulatory stringency and alignment with global GMP standards are pushing manufacturers to adopt MES for electronic batch records, data integrity, and audit compliance. The rise of smart factories, combined with substantial government incentives for digital transformation and increasing investment in Industry 4.0 technologies, further accelerates adoption. Additionally, the growth of CDMOs, large-scale capacity expansion, and the need for real-time visibility, traceability, and process optimization are fueling strong MES demand across the region.
REGION
China is expected to be the fastest-growing country across the pharmaceutical MES market during the forecast period
China is expected to record the fastest growth rate in the pharmaceutical MES market, driven by the rapid expansion of its biopharmaceutical and generics manufacturing ecosystem. The country’s aggressive push toward digitalizing production lines, supported by national initiatives such as Made in China 2025, is accelerating the adoption of MES across both state-owned and private pharmaceutical firms. The rising regulatory emphasis on data integrity, batch traceability, and compliance with global standards, such as GMP and FDA 21 CFR Part 11, is compelling manufacturers to shift from manual or semi-automated systems to integrated MES platforms. Additionally, China’s substantial investments in smart factories, robotics, AI, and IIoT are creating fertile ground for MES vendors to scale
pharmaceutical-manufacturing-execution-system-mes-market: COMPANY EVALUATION MATRIX
In the Pharmaceutical MES market matrix, companies such as Siemens and Dassault Systèmes (Star) lead with a strong market presence and a wide product portfolio, driving large-scale adoption across various applications in the pharmaceutical sector.
KEY MARKET PLAYERS
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
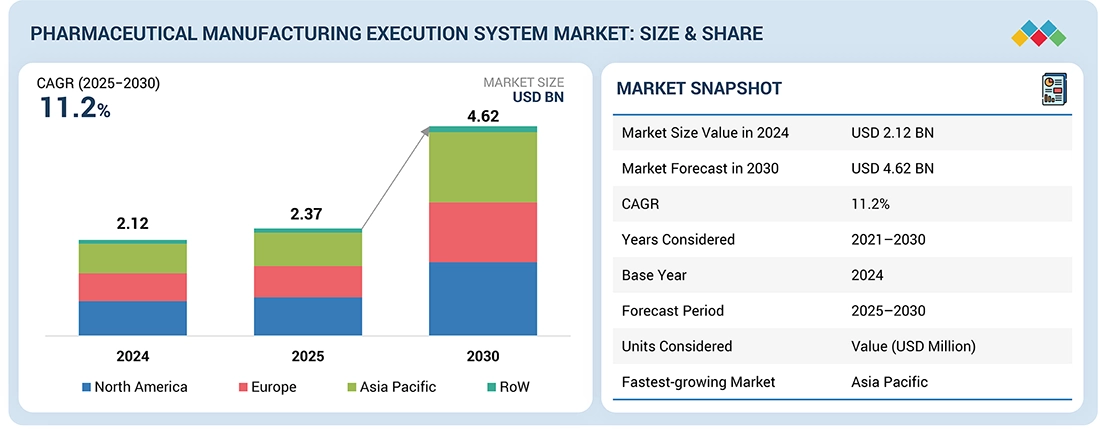
| Market Size in 2024 (Value) | USD 2.12 Billion |
| Market Forecast in 2030 (Value) | USD 4.62 Billion |
| Growth Rate | CAGR of 11.2% from 2025–2030 |
| Years Considered | 2021–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Billion/Million) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered | By Offering: Software and Services By Deployment: On-premises, Cloud and Hybrid |
| Country Scope | North America, Europe, Asia Pacific, RoW |
WHAT IS IN IT FOR YOU: pharmaceutical-manufacturing-execution-system-mes-market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| India-based Pharmaceutical & Biopharma Firm |
|
|
| US-based Drug Multisite Producer |
|
|
| China-based Industrial Equipment & Machinery OEM |
|
|
RECENT DEVELOPMENTS
- December2025. : Rockwell Automation announced a series of strategic innovations to its Manufacturing Execution System (MES) portfolio, focused on flexibility, scalability, and resiliency. This elastic MES portfolio is a cloud-native, interoperable MES platform designed to unify manufacturing operations across various industries, including pharmaceutical, food & beverage, and other industries.
- September 2025. : Körber announced the release of the new PAS-X MES 3.4, a robust upgrade to its Manufacturing Execution System (MES) that will facilitate the digital transformation of pharmaceutical manufacturing and life science applications. It brings modern UI/UX, AI-based user support, and intelligent lifecycle management, all aimed at faster, flexible shop-floor operations and regulatory compliance.
- January 2024. : Siemens launched manufacturing execution systems (MES) software. Powered by Mendix’s low-code technology, this software empowers manufacturers with unprecedented agility. This innovative solution redefines manufacturing across diverse industries.
Table of Contents

Methodology
The study involved four major activities in estimating the current size of the manufacturing execution systems market. Exhaustive secondary research has been done to collect information on the market, peer market, and parent market. To validate these findings, assumptions, and sizing with industry experts across the value chain through primary research has been the next step. Both top-down and bottom-up approaches have been employed to estimate the complete market size. After that, market breakdown and data triangulation methods have been used to estimate the market size of segments and subsegments. Two sources of information—secondary and primary—have been used to identify and collect information for an extensive technical and commercial study of the manufacturing execution systems market.
Secondary Research
Various secondary sources have been referred to in the secondary research process to identify and collect information important for this study. The secondary sources include annual reports, press releases, and investor presentations of companies; white papers; journals and certified publications; and articles from recognized authors, websites, directories, and databases. Secondary research has been conducted to obtain key information about the industry’s supply chain, the market’s value chain, the total pool of key players, market segmentation according to industry trends (to the most granular level), regional markets, and key developments from market- and technology-oriented perspectives. The secondary data has been collected and analyzed to determine the overall market size, further validated by primary research.
Primary Research
Extensive primary research was conducted after gaining knowledge about the current scenario of the manufacturing execution systems market through secondary research. Several primary interviews were conducted with experts from the demand and supply sides across four major regions—North America, Europe, Asia Pacific, and Rest of the World. This primary data was collected through questionnaires, emails, and telephonic interviews.
Market Size Estimation
Both top-down and bottom-up approaches have been used to estimate and validate the total size of the manufacturing execution systems market. These methods have also been used extensively to estimate the size of various subsegments in the market. The following research methodology has been used to estimate the market size:
- Major players in the industry and markets have been identified through extensive secondary research.
- The industry’s value chain and market size (in terms of value) have been determined through primary and secondary research processes.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Data Triangulation
After arriving at the overall size of the manufacturing execution systems market from the market size estimation process explained above, the total market has been split into several segments and subsegments. Data triangulation and market breakdown procedures have been employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments of the market. The data has been triangulated by studying various factors and trends from both the demand and supply sides. Along with this, the market size has been validated using both top-down and bottom-up approaches.
Market Definition
The Manufacturing Execution Systems (MES) market refers to software designed to maximize real-time factory production management by integrating operational technology (OT) with information technology (IT). Unlike enterprise resource planning (ERP) systems, MES offers granular visibility, control, and coordination of the manufacturing process at the shop floor level. These systems maximize production efficiency through optimized monitoring, quality assurance, workflow automation, and compliance with regulatory requirements.
Driven by Industry 4.0, smart manufacturing, and the need for real-time analytics, MES solutions enable manufacturers to minimize downtime, enhance agility, and optimize resource utilization. Industries such as automotive, electronics, pharmaceuticals, aerospace, and food & beverage depend on MES for smooth production, supply chain synchronization, and improved decision-making.
Key Stakeholders
- Associations and Regulatory Authorities (especially responsible for developing standards related to plant maintenance)
- Government Bodies, Venture Capitalists, and Private Equity Firms
- Automation Consultants
- Automation System Integrators
- ERP Developers
- MES Distributors and Providers
- Process and Discrete Industries
- Research Organizations and Consulting Companies
- Technology Providers
- Technology Investors, Standards Organizations, Forums, Alliances, and Associations
Report Objectives
- To describe, segment, and forecast the overall manufacturing execution systems market, by deployment mode, offering, application, and industry, in terms of value.
- To describe and forecast the market for four key regions: North America, Europe, Asia Pacific, and the Rest of the World (RoW), along with their respective countries, in terms of value.
- To describe the applications and leveraging entities of manufacturing execution systems and software.
- To provide an overview of the recent trends in the market.
- To provide detailed information regarding the drivers, restraints, opportunities, and challenges influencing the growth of the market.
- To analyze the supply chain, trends/disruptions impacting customers' businesses, market/ecosystem maps, pricing analysis, and the regulatory landscape pertaining to pharmaceutical manufacturing execution systems market
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall market size.
- To analyze opportunities in the market for stakeholders and provide a competitive landscape of the market.
- To strategically profile the key players and comprehensively analyze their market positions in terms of their ranking and core competencies, along with detailing the competitive landscape for market leaders,
- To analyze competitive developments, such as product launches, acquisitions, collaborations, agreements, and partnerships, in the manufacturing execution systems market.
- To provide ecosystem analysis, case study analysis, patent analysis, technology analysis, value chain analysis, trends/disruptions impacting customer business, the impact of AI/Gen AI, key conferences and events, pricing analysis, Porter’s five forces analysis, and regulations pertaining to the market under study.
- To provide a macroeconomic outlook based on all the regions in the region chapter.
Available Customizations:
With the given market data, MarketsandMarkets offers customizations according to the company’s specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Pharmaceutical Manufacturing Execution System (MES) Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Pharmaceutical Manufacturing Execution System (MES) Market