
US Veterinary Vaccines Market Size, Growth, Share & Trends Analysis
US Veterinary Vaccines Market by Type (Porcine, Poultry, Livestock, Companion Animals), Technology (Live Attenuated, Inactivated, Toxoid), Route of Administration (Intramuscular, Subcutaneous, Oral), and End User (Hospitals, Clinics) – Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
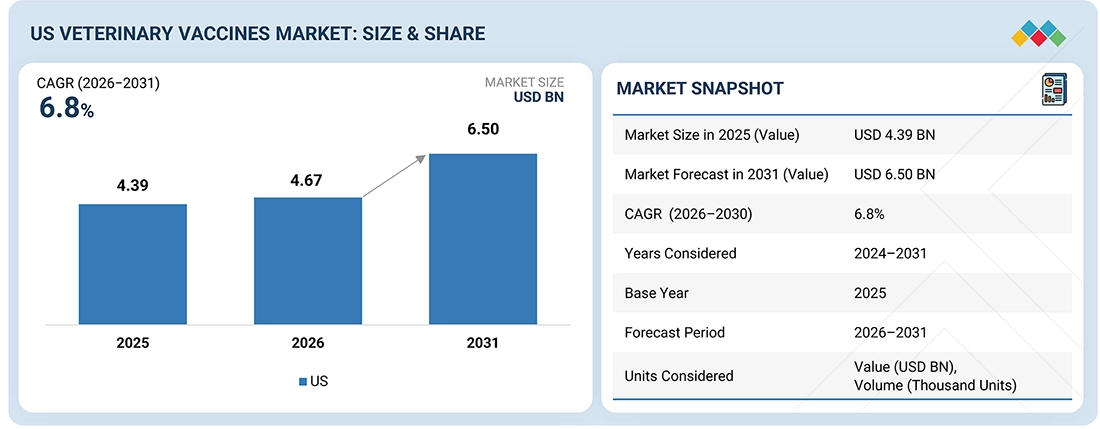
The US veterinary vaccines market, valued at US$4.39 billion in 2025, stood at US$4.67 billion in 2026 and is projected to advance at a resilient CAGR of 6.8% from 2026 to 2031, culminating in a forecasted valuation of US$6.50 billion by the end of the period. This growth is attributed to the increasing awareness regarding animal health as well as the rising pet population and pet ownership. Other emerging trends affecting the growth of this segment are government initiatives to improve the health of animals and increased adoption of pet coverage. Further, the continuous focus on developing novel and advanced vaccines coupled with increasing application of these vaccines is expected to propel market growth.
KEY TAKEAWAYS
-
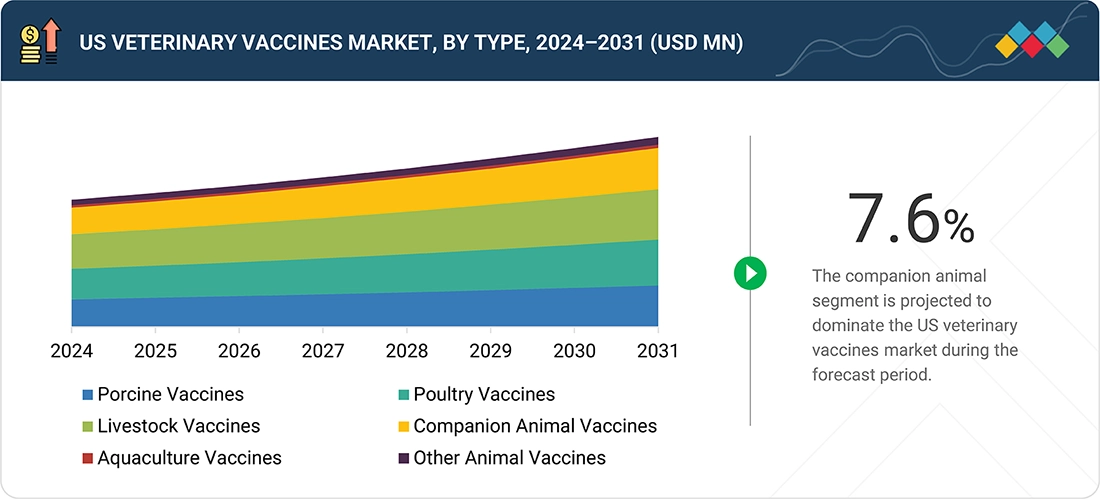
By TypeBy type, the livestock vaccines segment accounted for the largest share (31.8%) in 2025.
-
By DiseaseBy disease, the livestock vaccines segment accounted for the largest share (31.5%) in 2025.
-
By TechnologyBy route of administration, the subcutaneous route of administration accounted for largest share.
-
By Route of AdministrationBy technology, the live attenuated vaccines segment accounted for the largest share (45.4%) of the US veterinary vaccines market in 2025.
-
Competitive LandscapeZoetis, Merck & Co, Inc., and Boehringer Ingelheim International GmbH are identified as STAR players in the market. These industry leaders have very broad vaccine portfolios, large-scale manufacturing, strong R&D, and global distribution.
-
Competitive LandscapeHIPRA, Hester Biosciences, and Vaxxinova are identified as high-growth innovators/SMEs. These companies are carving out niches through regional specialization, novel vaccine technologies.
Increased pet ownership, increasing importance of animal welfare, and rising incidence of infectious and zoonotic diseases in the region have led to a steady growth in the US veterinary vaccines market. Strong regulatory frameworks and government-driven animal health initiatives in the region promote widespread vaccination among companion animals and livestock. The demand for effective immunization solutions is further driven by the adoption of sustainable livestock practices and increasing stringent food safety standards. However, one potential hindrance to market growth could be high costs associated with development.
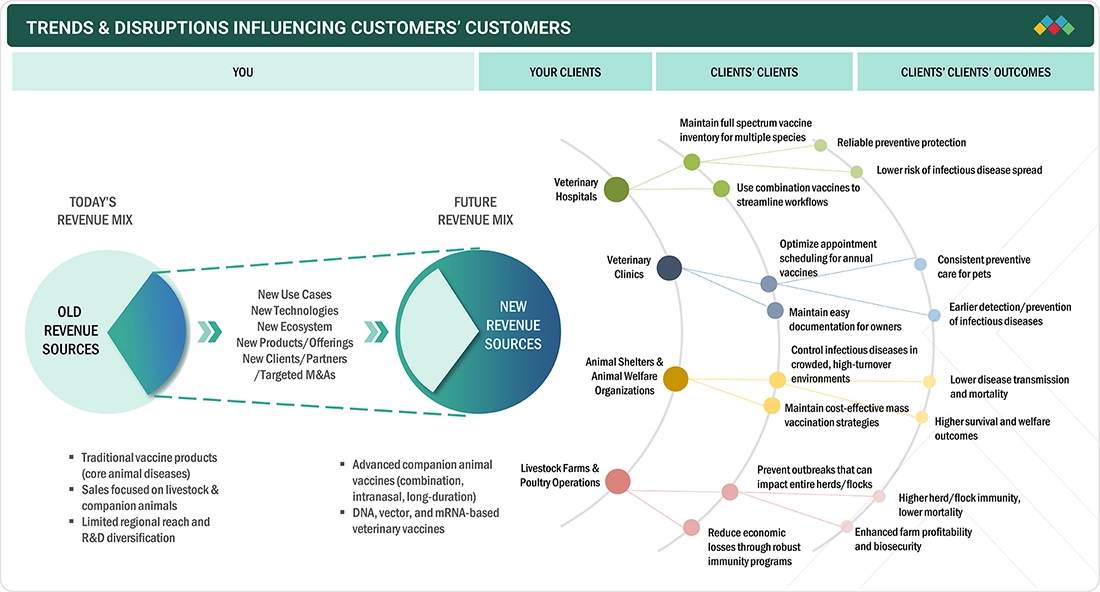
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The US veterinary vaccines market is subject to a transformational wave owing to evolving disease patterns in pets need for preventive animal health. Additionally, advancements in vaccine technologies would improve convenience in usage, widen the scope of protection, and prolong its duration. Combination, vector-based, and long-acting vaccines will play a key role in such a trend. Biosecurity concerns become more serious, regulatory restrictions on vaccines become more stringent, and cost-effective large-scale immunization is implemented on the farm and in shelters in relation to procurement and use. The adoption of more digital health tools as well as better tracking of vaccines and wider distribution partnerships create added layers to how vaccines are delivered and monitored. Overall, this market in the US is shifting from traditional vaccines to more ground-breaking, tech-driven, and cheaper immunization solutions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rise in companion animal population and pet ownership

-
Increasing incidence of infectious zoonotic diseases
Level
-
High cost of vaccine development
Level
-
Technological advancements for veterinary vaccine development
Level
-
Stringent regulatory requirements for licensing veterinary vaccines
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
US VETERINARY VACCINES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Broad vaccine portfolio for livestock (avian influenza, bovine respiratory disease, swine fever) and pets (rabies, parvovirus) | Strong R&D in recombinant/vector vaccines | Improved livestock productivity, reduced outbreak losses, stronger food security, enhanced pet health |
 |
Innovative platforms, including RNA-particle and recombinant vaccines| Targeting zoonotic diseases and production animals | Broader protection spectrum, longer shelf life, better safety profile | Supports One Health by reducing zoonotic risks |
 |
Poultry and swine vaccines and companion animal lines | Focus on global emerging disease threats | Reduced flock/herd mortality, improved farmer profitability, expanded vaccine access in developing regions |
 |
Expanding livestock and companion animal vaccines with scalable production capacity | Greater vaccine availability in emerging markets, better farmer incomes, growth in preventive pet care |
 |
Companion animal vaccines with new formulations and delivery routes | Higher pet vaccination compliance, improved household disease prevention, convenient administration |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The US veterinary vaccines market ecosystem includes vaccine manufacturers, such as Zoetis, Merck, Boehringer Ingelheim, Elanco, Virbac and Ceva; regulatory bodies that determine safety and effectiveness, cold-chain logistics that maintain their quality, and research and academic institutions that further innovations in the next generation; and end users, such as veterinary hospitals and clinics, the ultimate consumers of these vaccines.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The livestock vaccines segment is projected to be the largest segment in the animal vaccines market during the forecast period. Livestock animals are owned for the production of meat or milk. With the increasing global population, the demand for animal-derived food products is on the rise, thus leading to intensified livestock farming to fulfill these requirements. The overall meat consumption is increasing because of improved production and rising demand, both domestic and global. According to a Carlisle technology, in 2023, the US is the largest beef producer in the world. About 30% of its farms are being used for beef production. As livestock farming becomes more industrialized and regulated, it will further require the vaccines for disease prevention.
The live attenuated vaccines segment is projected to lead the market during the forecast period. The ease of administration and long-term immune response availability of these vaccines are some of the major drivers behind this growth. Live attenuated vaccines are very effective, cost-efficient, and offer protection against a variety of serious diseases in livestock. The role of these vaccines in maintaining healthy disease-free livestock, especially in regions of a highly intensive trade in animal farming, is further expected to drive the growth of the segment in the coming years.
The veterinary hospitals segment is projected to lead the market during the forecast period driven by growing pet ownership and the increasing focus on preventive animal health care. The demand for animal-derived food products and growing livestock activities further compel the need for effective vaccination programs and disease management. Veterinary hospitals act as primary healthcare providers that have advanced diagnostic, treatment, and vaccination services for companion animals and livestock. The bulk of veterinary vaccine manufacturers practicing in the US prefer to either market their goods directly to licensed veterinarians or sell them through proprietary distributors providing their services exclusively to veterinary professionals rather than third-party pharmacies. This enhances the role of veterinary hospitals, increases patient visits, and supports professional advocacy for animal health awareness. Moreover, growing spending on animal care keeps boosting the vaccination rate within veterinary hospitals in the US.
US VETERINARY VACCINES MARKET: COMPANY EVALUATION MATRIX
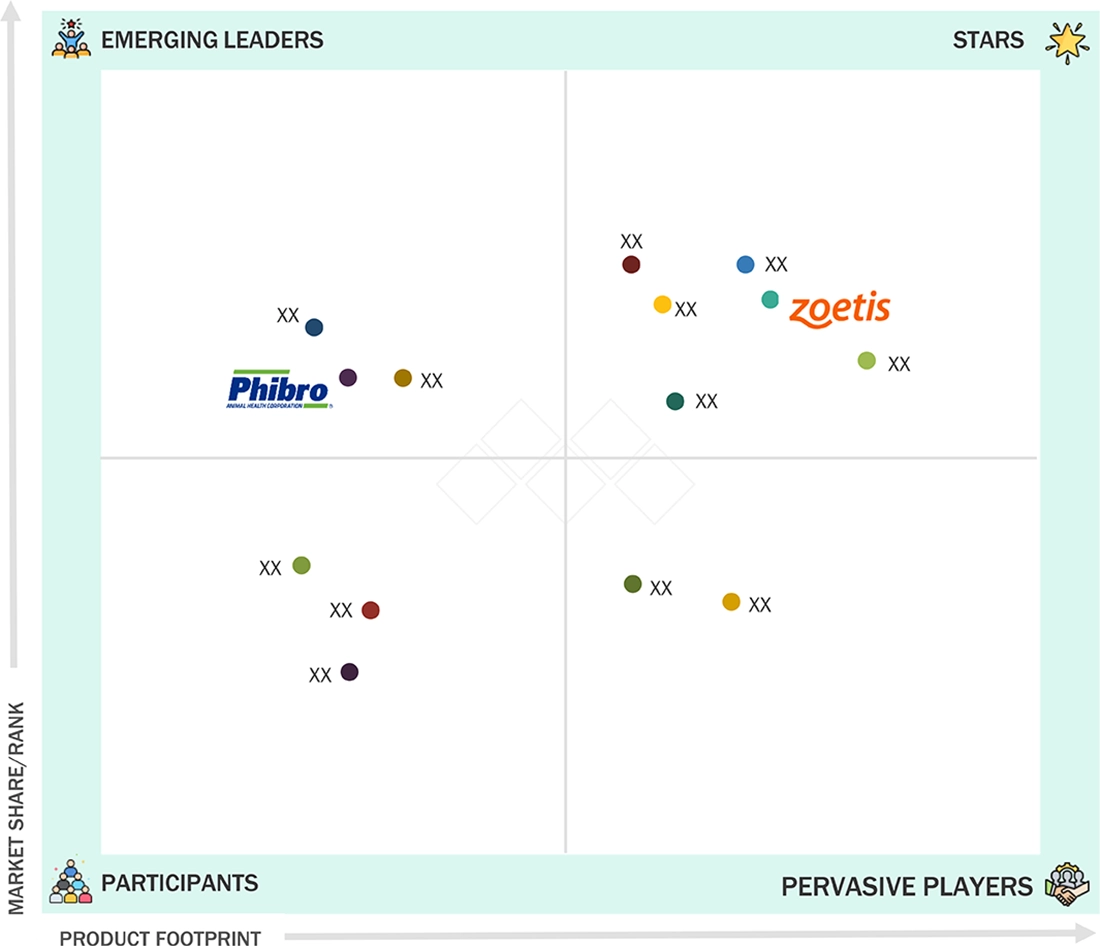
In the US veterinary vaccines market's evaluation matrix, Zoetis (Star) is the global leader with a strong presence around the globe, a wide-ranging vaccine portfolio, and technologies advanced in application of its vaccines to livestock and companion animal vaccines, assuring rapid acceptance among commercial farms and veterinary practices. Phibro Animal Health (Emerging Leader) is now on an accelerated path with innovative vaccine solutions that are cost-effective, the emphasis being laid on emerging markets as well as expanding its footprints in animal health.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Zoetis (US)
- Merck & Co, Inc. (US)
- Boehringer Ingelheim International GmbH (Germany)
- Elanco (US)
- Virbac (France)
- Ceva (France)
- Phibro Animal Health Corporation (US)
- Hester Biosciences Limited (India)
- Neogen Corporation (US)
- HIPRA (Spain)
- Vaxxinova International B.V. (Netherlands)
- Endovac Animal Health (US)
- Brilliant Bio Pharma Pvt Ltd (India)
- Aptimmune Biologics, Inc. (US)
- Torigen Pharmaceuticals, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 4.39 Billion |
| Market Size in 2031 (Value) | USD 6.50 Billion |
| Growth Rate | CAGR of 6.8% from 2026–2031 |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD Billion), Volume (Thousand Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | US |
| Parent & Related Segment Reports |
Veterinary Vaccines Market Europe Veterinary Vaccines Market |
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Detailed evaluation of veterinary vaccines by type (live attenuated, inactivated, recombinant, etc.), disease target (porcine, poultry, livestock, companion animals, aquaculture), and technology | Offer insights on market trends, emerging vaccine technologies, and efficacy comparisons to support informed decision-making in the US |
| Company Information | Profiles of leading players, including Zoetis, Merck Animal Health, Elanco, Boehringer Ingelheim, and Phibro, highlighting product portfolios, R&D pipelines, and market presence | Identify strategic partnerships, licensing agreements, USDA approvals, manufacturing expansions, and mergers & acquisitions shaping the competitive landscape of the U.S. veterinary vaccines market |
| Geographic Analysis |
|
|
RECENT DEVELOPMENTS
- November 2025 : Boehringer Ingelheim International GmbH launched EURICAN L4 vaccine to protect dogs against the growing threat of leptospirosis, a severe and re-emerging infectious disease.
- November 2024 : Ceva invested in a new vaccine manufacturing plant in Monor, Hungary. This plant would be equipped with the most advanced technologies. In this plant, Ceva is expected to produce fermentation-based multicomponent inactivated vaccines for animals that will help expand its global production capacity.
- September 2024 : Merck & Co., Inc. announced the expansion of its NOBIVAC NXT platform with the launch of a groundbreaking new vaccine for Feline Leukemia Virus (FeLV). This vaccine is the first and only to incorporate RNA-Particle Technology, offering a novel alternative for feline FeLV prevention.
- March 2024 : Zoetis announced the purchase of a manufacturing site in Melbourne, Australia, to significantly expand its current operations at the site and increase future capabilities to develop and manufacture vaccines for sheep, cattle, dogs, cats and horses.
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, fundamental market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), American Veterinary Medical Association (AVMA), Animal Health Institute (AHI), National Animal Health Laboratory Network (NAHLN)And Food and Agriculture Organization of the United Nations (FAO)were referred to identify and collect information for the global US veterinary vaccines market study. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the US veterinary vaccines market. The primary sources from the demand side include hospitals & clinics, physiotherapy clinics and home care settings. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
For the global market value, annual revenues were calculated based on the revenue mapping of major product manufacturers and OEMs active in the global US veterinary vaccines market. All the major product manufacturers were identified at the global and/or country/regional level. Revenue mapping for the respective business segments/sub-segments was done for the major players (who contribute at least 70-75% of the market share at the global level). Also, the global US veterinary vaccines market was split into various segments and sub-segments based on:
- List of major players operating in the US veterinary vaccines products market at the regional and/or country level
- Product mapping of various US veterinary vaccines manufacturers at the regional and/or country level
- Mapping of annual revenue generated by listed major players from US veterinary vaccines (or the nearest reported business unit/product category)
- Revenue mapping of major players to cover at least 70-75% of the global market share as of 2023
- Extrapolation of the revenue mapping of the listed major players to derive the global market value of the respective segments/subsegments
- Summation of the market value of all segments/subsegments to arrive at the global point-of-care diagnostics market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
Market Size Estimation (Bottom Up approach & Top down approach)

Data Triangulation
After arriving at the overall size of the global US veterinary vaccines market through the above-mentioned methodology, this market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value data for the key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Market Definition
A veterinary vaccine is a biological preparation that provides immunity by stimulating animal’s immune system to recognize and combat specific pathogen. The US veterinary vaccines market serves a range of vaccines such as live attenuated, inactivated, recombinant, and toxoid vaccine for a wide range of animals such as poultry, livestock, porcine, aquaculture, and companion animals. Vaccination aids in improving the health of animals, reducing the outbreak of zoonotic diseases, supporting higher productivity, and help securing economic stability.
Stakeholders
- Animal vaccine manufacturers
- Animal healthcare product manufacturers
- Animal vaccine distributors and wholesalers
- Animal welfare associations
- Veterinary clinics and care centers
- Research and consulting firms
- Veterinary Research and Development Organizations
- Contract research organizations
- Contract manufacturing organizations
- Venture capitalists
Report Objectives
- To define, measure, and describe the global US veterinary vaccines market by type, disease, technology, route of administration, end user
- To provide detailed information about the major factors influencing the market growth (drivers, restraints, challenges, and opportunities)
- To strategically analyze the regulatory scenario, pricing, value chain analysis, supply chain analysis, ecosystem analysis, technology analysis, Porter’s Five Forces analysis, pipeline analysis and patent analysis
- To analyze micromarkets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To forecast the size of market segments in North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa
- To strategically analyze the market structure, profile the key players in the global US veterinary vaccines market, and comprehensively analyze their core competencies
- To track and analyze company developments such as acquisitions, partnerships, expansions, and product launches and approvals in the US veterinary vaccines market
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the US Veterinary Vaccines Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in US Veterinary Vaccines Market