
Europe Enteral Feeding Devices Market Size, Growth, Share & Trends Analysis
Europe Enteral Feeding Devices Market by Type [Tubes(Nasal, Gastrostomy), Pumps {Portable, Stationary}, Enteral Syringe, Administration Set, Consumable], Insertion Method, Age Group (Neonate, Pediatric, Geriatric), Application, End user- Forecast to 2032




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
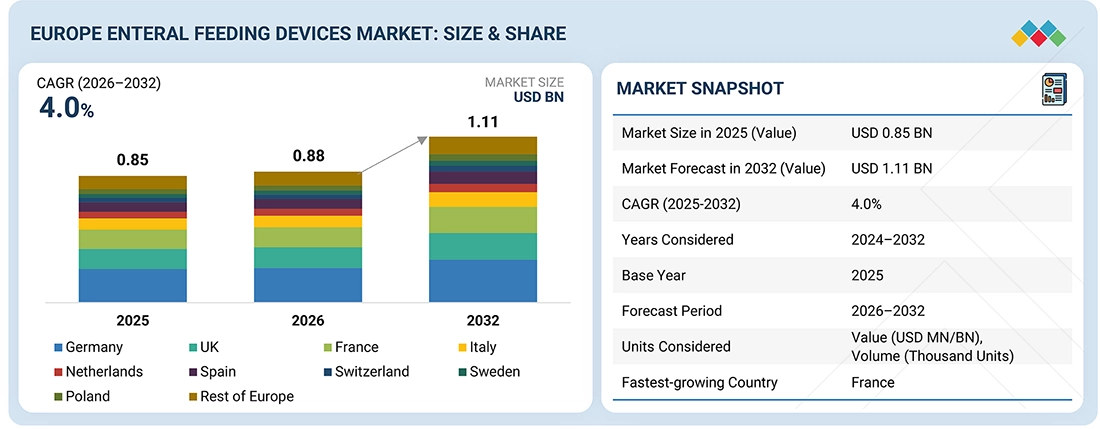
The Europe enteral feeding device market, valued at USD 0.85 billion in 2025, stood at USD 0.88 billion in 2026 and is projected to advance at a resilient CAGR of 4.0% from 2026 to 2032, culminating in a forecasted valuation of USD 1.11 billion by the end of the period. This is due to the increasing requirements for clinical nutrition, rising hospitalizations, and the senior demographics and advanced healthcare infrastructure in that region.
KEY TAKEAWAYS
-
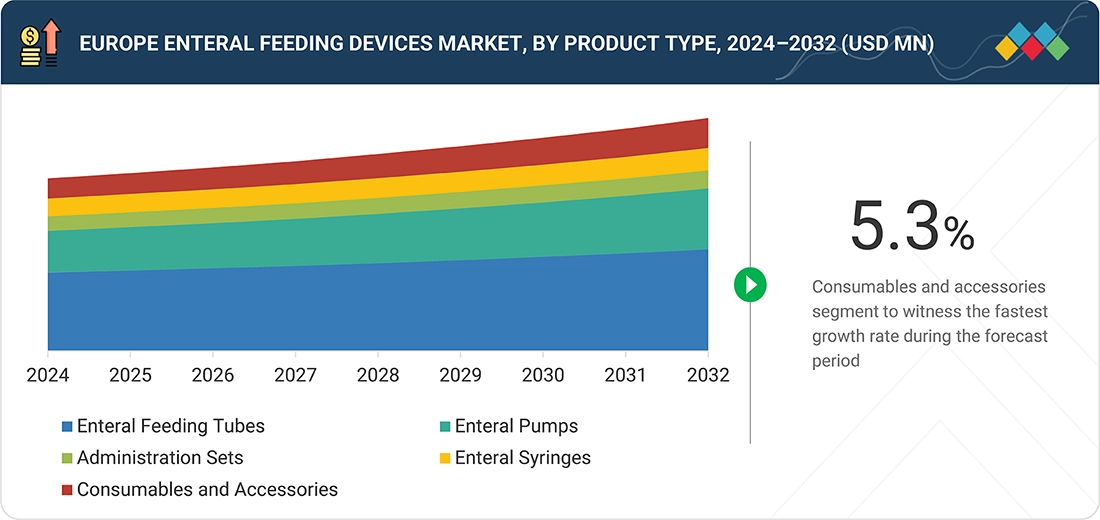
BY TYPEBy type, the enteral feeding tubes segment is expected to account for the largest share of 45.4% in 2025.
-
BY INSERTION METHODThe Transnasal, Transoral, and Percutaneous Endoscopic Insertion (PEG/PEJ) segment has accounted for the largest share of 67.1% in 2024. It is expected to witness the highest CAGR of 4.4% during the forecast period.
-
BY AGE GROUPThe neonatal patients segment is expected to witness the highest growth rate of 4.2% during the forecast period in this market.
-
BY APPLICATIONThe oncology segment has accounted for the largest share of 26.2% in 2024 in the Europe enteral feeding devices market, by application.
-
BY END USERThe home care settings segment is projected to have the highest CAGR of 4.7% from 2026 to 2032 in the Europe enteral feeding devices market, by end user.
-
BY COUNTRIESFrance is expected to grow at the highest CAGR of 4.7% during the forecast period from 2026 to 2032 in the Europe enteral feeding devices market.
-
COMPETITIVE LANDSCAPE - MAJOR PLAYERSFresenius Kabi (Germany) and Nestlé S.A. (Switzerland) are recognized as star players in the Europe enteral feeding devices market due to their extensive product portfolios, strong regional presence, and deep healthcare channel penetration.
-
COMPETITIVE LANDSCAPE - STARTUP/SMEAmong startups and SMEs, Hoist-Medical (France) and Luminate Medical (Ireland) stand out as progressive companies, gaining attention for their innovative approaches and growing influence within the Europe enteral feeding devices landscape.
The Europe enteral feeding devices market is expanding at an even pace, driven by a mature healthcare environment, government support, and increased emphasis on clinical nutrition. The region has maintained a substantial share in overall market revenues, primarily because of its aged population as well as diseases requiring enteral care. Germany, France, and the UK are identified as major adopters in hospitals and home care settings.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
In Europe, enteral devices are undergoing a transition, with a growing emphasis on patient- and port-friendly pumps, low-profile gastrostomy devices, and ENFit-compatible connectors to make them safer. The industry is developing for home care, while a unified regulatory framework, such as the EU MDR, is also contributing to improvements in device standards. The home enteral nutrition program and sustainable device acquisition are also gaining importance.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing Prevalence of Chronic and Critical Illnesses

-
Growing Geriatric Population
Level
-
Complications associated with Enteral Feeding Devices
-
Regulatory Compliance and Product Recalls
Level
-
Growing Awareness and Training Programs
-
Emerging Market Penetration
Level
-
Shortage of Intubation Specialists
-
Insufficient Reimbursement Policies in Emerging Economies
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing Prevalence of Chronic and Critical Illnesses
Rising incidence rates of chronic diseases such as cancer, neurological disorders, stroke, COPD, and gastrointestinal disorders in Europe are thus fueling the enteral feeding devices market. Aging, particularly in Western Europe, is contributing towards increased rates of hospitalizations and long-term care facility admissions, and thus, there are high rates of dysphagia and malnutrition. Established health care infrastructure and government-funded health care services in hospitals, rehab centers, and long-term care facilities are thus fueling the enteral feeding devices market.
Restraint: Complications associated with Enteral Feeding Devices
In Europe, issues such as blockage of the tube, aspiration pneumonitis, infection at the site of the tube insertion, and intolerance to feeding continue to pose a challenge to older and home-care patients. Clinical practices may also vary between regions and the level of training of caregivers; hence, there may be potential for incorrect placement and care of the tube. In the EU, strict regulatory control and surveillance can pose a barrier to updates and adoption due to issues involving safety concerns.
Opportunity: Growing Awareness and Training Programs
In Europe, there are opportunities for players due to enhanced understanding of clinical nutrition and training programs among health care professionals. Nutritional societies in individual European countries, ESPEN recommendations, and educational programs by hospitals are all contributing toward improved standardization in enteral nutrition administration. Development in home-based Enteral Nutrition (HEN), where community nursing facilities and payment options are available in some European countries, such as Germany, France, and the UK, is also stimulating demand for sophisticated and user-friendly enteral feeding systems.
Challenge: Shortage of Intubation Specialists
Several European countries have a shortage of trained healthcare professionals who are skilled in the placement and management of enteral tubes, particularly in rural areas and long-term care settings. Workforce pressures within the public healthcare systems and an increase in volumes of patients can delay the initiation of enteral nutrition. Although reimbursement systems are generally strong, the variance in national policies and procedural coverage will add complexity and may challenge consistent and timely access to enteral feeding services throughout Europe.
EUROPE ENTERAL FEEDING DEVICES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Manufactures a full range of enteral feeding pumps, tubes, and nutrition formulas for hospital and home care settings, ensuring safe enteral delivery for critically ill and long-term patients | Reliable nutritional support| Reduced feeding interruptions, and enhanced patient recovery outcomes through integrated feeding systems |
 |
Offers a comprehensive range of enteral feeding solutions under Nestlé Health Science, including clinical-grade pumps, pediatric-specific enteral tubes with ergonomic designs, and anti-reflux features for safe nutritional delivery in both hospital and home care environments | Enhanced delivery accuracy| Greater patient comfort and flexibility of use in diverse care settings with tailored nutrition options |
 |
Supplies complete enteral feeding system portfolios such as ENFit-compliant feeding tubes, ambulatory and syringe pumps, and accessory sets designed for neonates, pediatric, and adult patients in intensive care and home care | Improved patient safety with standardized connectors| Reduced risk of misconnections and versatile device compatibility for varied clinical needs |
 |
Supplies specialized medical nutrition products and enteral tubes designed for disease-specific conditions, including oncology, neurology, and geriatrics | Supports better clinical outcomes| Maintains nutritional status| Complements enteral feeding hardware for complete nutritional therapy |
 |
Develops enteral feeding syringes, pumps, tubes, and administration sets optimized for precision and hygiene in intensive care and home care setups | Enhanced dosing accuracy| Robust build for hospital-grade use| Improved infection prevention with closed feeding systems |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe enteral feeding devices market operates in a highly integrated and structured healthcare infrastructure. Public healthcare providers remain pivotal in the process of promoting the adoption and usage of the service. Well-developed hospitals, cancer institutes, and long-term care facilities continuously employ the services of enteral nutrition products as part of their standard medical practice. Home-based enteral nutrition is additionally increasing in popularity.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe enteral feeding devices market, by type
In Europe, the consumables & accessories segment is expected to register the highest growth rate in the enteral feeding devices market, as it is a cyclical process and a long-term therapy. Feeding tubes, administration sets, connectors, and syringes are frequently changed in a hospital setting to maintain asepsis or infection control. An increasing number of home enteral nutrition therapy programs, an aging population, and infection-control practices in European healthcare facilities are factors pushing the consumption level of these products upward.
Europe enteral feeding devices market, by insertion method
Transnasal, transoral, and percutaneous endoscopic insertion (PEG/PEJ) segment leads the market due to clinical efficacy and comfort for the patients. PEG/PEJ procedures combine long-term enteral access with minimally invasive techniques, making them widely favored both in hospitals and for home care settings. Their established usage in intensive care, oncology, and neurology wings propels this segment to dominance.
Europe enteral feeding devices market, by age group
The neonatal patients market is also forecasted to expand at the highest growth rate, due to an increase in survival rates among preterm neonates and because these neonates have partially developed feeding capabilities. Therefore, they often require tube feeding. Consequently, there will be a demand for small, specific enteral devices for neonates.
Europe enteral feeding devices market, by application
In Europe, oncology is a large area of application because of the substantial burden of cancer, along with the widespread integration of nutrition therapy into cancer management. Malnutrition, dysphagia, and cachexia, as part of supportive care, are integral components of standard management practices in European oncology institutes. The incorporation of enteral nutrition into palliative care is also encouraged by guidelines developed by the European Society for Clinical Nutrition and Metabolism.
Europe enteral feeding devices market, by end user
The home care environment is expected to witness the highest growth rate in the Europe region due to the increasing adoption of home enteral nutrition programs supported by public healthcare systems. Countries such as Germany, France, the UK, and the Scandinavian countries prefer early discharge from hospitals and the adoption of community care for their patients with neurological and chronic illnesses. Reimbursement policies, the availability of qualified home care nurses, and the use of portable pumps and simplified tube feeding devices are making it more acceptable for the adoption of long-term enteral nutritional care in the home environment.
REGION
Enteral feeding devices market, by country
The presence of chronic conditions, such as cancer, stroke, and gastrointestinal disorders that restrict oral intake and increase reliance on alternative nutritional support, has resulted in increased demand. The high prevalence of chronic diseases necessitates more conservative management with enteral nutrition, an increasing population of geriatric patients with difficulty swallowing, the widespread adoption of feeding pumps and tubes, a robust healthcare infrastructure, and supportive policies for home-based care, all of which drive the growth of the Europe enteral feeding devices market.
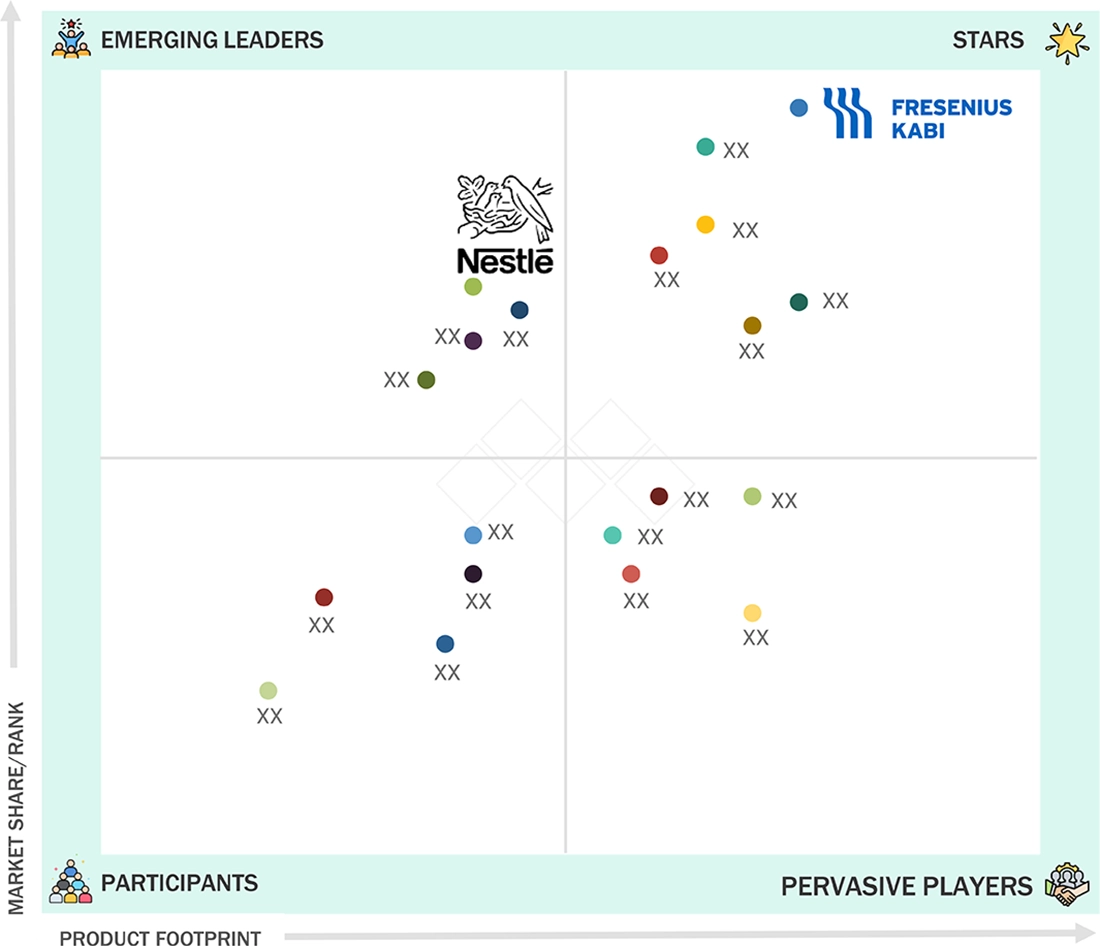
EUROPE ENTERAL FEEDING DEVICES MARKET: COMPANY EVALUATION MATRIX
In the Europe enteral feeding devices market, Fresenius Kabi (Star) has a strong product portfolio, deep presence across hospitals and home care, and a well-established manufacturing & distribution network in Europe. Nestlé (Emerging Leader) has been expanding its solutions of home enteral nutrition and increasing collaboration with healthcare providers to support steady growth.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- B. Braun SE (Germany)
- Fresenius Kabi AG (Germany)
- Danone S.A. (France)
- Nestlé (Switzerland)
- Vygon S.A. (France)
- Smiths Medical (UK)
- Medtronic plc (Ireland)
- Conmed Corporation (US)
- Fresenius Kabi (Germany)
- Cair LGL (France)
- Nutricia Medical (Netherlands)
- GBUK Group (UK)
- Medicina Ltd (UK)
- Cardinal Health (US)
- Avanos Medical (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 0.85 Billion |
| Market Forecast in 2032 (Value) | USD 1.11 Billion |
| CAGR (2025-2032) | 4.0% |
| Years Considered | 2024–2032 |
| Base Year | 2025 |
| Forecast Period | 2026–2032 |
| Units Considered | Value (USD Million/Billion), Volume (Thousand Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, UK, France, Italy, Spain, Netherlands, Switzerland, Sweden, Poland, Rest of Europe |
| Parent & Related Segment Reports |
Enteral Feeding Devices Market US Enteral Feeding Devices Market LATAM Enteral Feeding Devices Market APAC Enteral Feeding Devices Market |
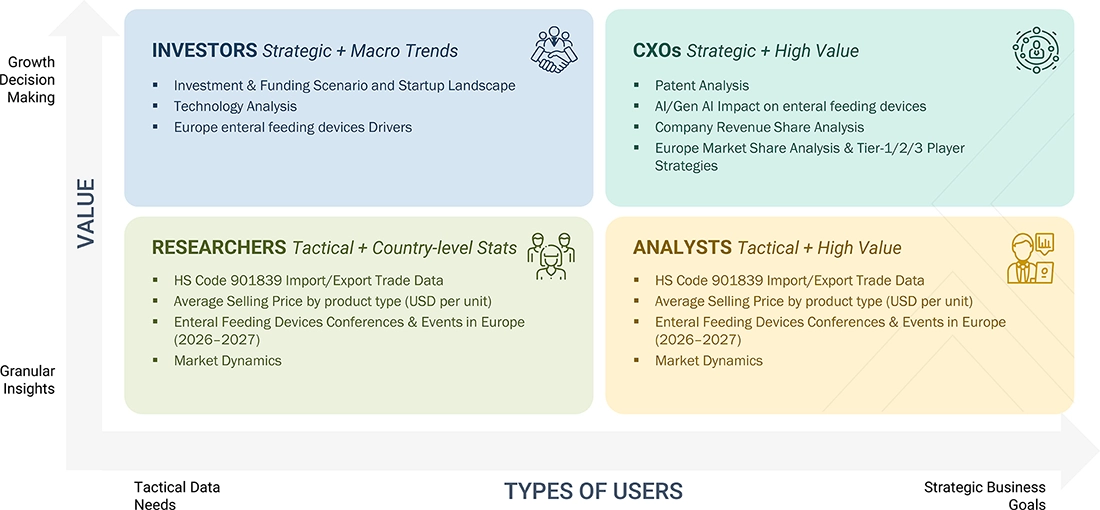
WHAT IS IN IT FOR YOU: EUROPE ENTERAL FEEDING DEVICES MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Detailed assessment of enteral feeding devices by type, application, age group, and insertion method | Analysis of emerging trends such as smart, connected pumps & remote monitoring, personalized nutrition & smart dosing, compact, portable and user-friendly pumps for home & ambulatory use, and low-profile & pediatric/neonatal specialized tubes |
| Company Information | Comprehensive profiles of major players such as Fresenius Kabi, Nestle, Danone, B Braun, and other companies | Identification of strategic partnerships, collaborations, licensing agreements, and mergers & acquisitions in Europe enteral feeding devices market |
| Geographic Analysis |
|
|
RECENT DEVELOPMENTS
- July 2025: Danone strengthened its medical and plant-based nutrition portfolio by acquiring a majority stake in Kate Farms, supporting innovation and cross-market synergies from its European base.
- March 2025: Fresenius Kabi announced an expansion with a new innovation center to advance enteral and patient-specific nutrition solutions for European and global markets.
- February 2024: Fresenius Kabi reinforced its leadership in clinical nutrition across Europe by extending its collaboration with ESICM to support education and professional development in intensive care nutrition.
- October 2023: Nestlé Health Science expanded its digitally enabled nutrition ecosystem through a collaboration with Amwell, aligning European nutrition expertise with global digital health capabilities.
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the comprehensive use of secondary sources, directories, databases (such as Factiva, Bloomberg Businessweek, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the Europe Enteral Feeding Devices Market. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. Moreover, a database of the key industry leaders was prepared using secondary research.
Primary Research
In the primary research process, various sources from the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing & sales directors, technology & innovation directors, and related key executives from various companies and organizations in Europe Enteral Feeding Devices Market. The primary sources from the demand side include medical OEMs, analytical instrument OEMs, CDMOs, and service providers. Primary research was conducted to validate the market segmentation, identify key players, and gather insights on industry trends & market dynamics.
Market Size Estimation
In this report, the size of the Europe Enteral Feeding Devices Market was determined using revenue analysis of leading players. For this purpose, key players in the market were identified, and their revenues from the enteral feeding device business were determined through various insights gathered during the primary and secondary research phases. Secondary research included the study of the annual and financial reports of the top market players. Meanwhile, primary research included extensive interviews with key opinion leaders, such as CEOs, directors, and marketing executives.
Segmental revenues were calculated based on the revenue mapping of major solution/service providers to calculate the market value. This process involved the following steps:
- Generating a list of major players operating in the Europe Enteral Feeding Devices Market
- Mapping annual revenues generated by major players in the Europe Enteral Feeding Devices Market (or nearest reported business unit/product category)
- Revenue mapping of key players to cover a major share of the market as of 2024
- Extrapolating the value of the Europe Enteral Feeding Devices Market
Data Triangulation
After arriving at the overall market size from the market size estimation process explained above, the Europe Enteral Feeding Devices Market was split into segments and subsegments. Data triangulation and market breakdown procedures were employed to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments. The data was triangulated by studying various factors and trends from both the demand and supply sides. Additionally, the Europe Enteral Feeding Devices Market was validated using top-down and bottom-up approaches.
Market Definition
Enteral feeding devices are instruments and configurations designed for nutrition administration directly into the gastrointestinal tract, bypassing the mouth. These devices serve medically challenged patients who cannot eat food orally due to swallowing disorders, severe illnesses, or surgical interferences. Enteral feeding can be accomplished through the insertion of tubes from the nose or mouth, which are then placed into the stomach or small intestine to deliver nutrients.
Stakeholders
- Manufacturers and Distributors of Enteral Feeding Devices
- Ambulatory Surgical Centers
- Home Care Facilities
- Healthcare Institutions (Hospitals and Cardiac Centers)
- Research Institutions
- Research and Consulting Firms
- Contract Research Organizations (CROS) and Contract Manufacturing Organizations (CMOS)
- Academic Medical Centers and Universities
- Market Research and Consulting Firms
- Clinical Research Organizations
- Group Purchasing Organizations (GPOs)
- Academic Medical Centers and Universities
- Accountable Care Organizations (ACOs)
Report Objectives
- To define, describe, and forecast the Europe Enteral Feeding Devices Market on product, age group, application, and end user
- To identify and analyze drivers, restraints, opportunities, and challenges influencing market growth
- To strategically analyze micro markets concerning individual growth trends, prospects, and contributions to the total market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To profile key market players and comprehensively analyze their market shares and core competencies
- To analyze competitive developments such as acquisitions, agreements, collaborations, expansions, partnerships, and product launches/approvals in the Europe Enteral Feeding Devices Market
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Enteral Feeding Devices Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Europe Enteral Feeding Devices Market