
Europe Pen Needles Market Size, Growth, Share & Trends Analysis
Europe Pen Needles Market By Type (Standard Pen Needles, Safety Pen Needles), Length (8mm, 5mm), Setting (Home Care Settings), Application (Glucagon-like Peptide-1 Therapy), Mode of Purchase (Over-the-counter Purchase, Online Purchase) - Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
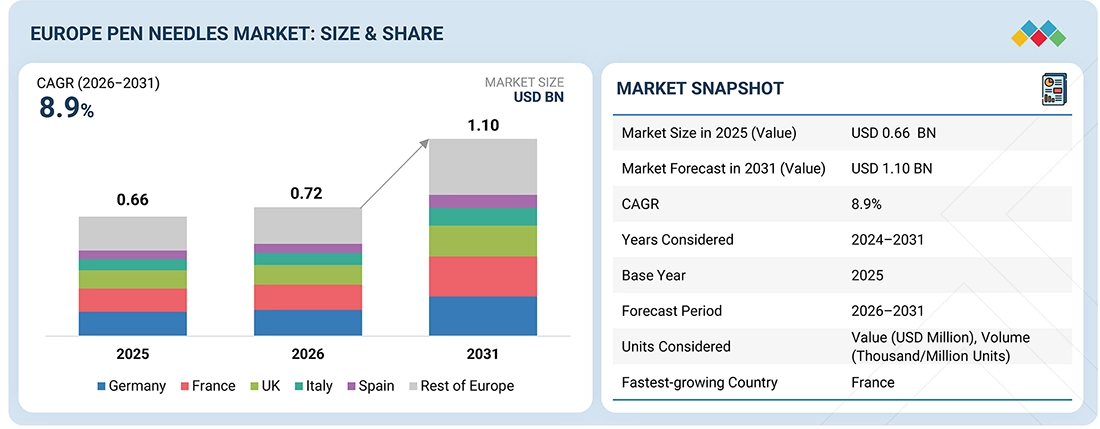
The global Europe pen needles market, valued at US$0.66 billion in 2025, stood at US$0.72 billion in 2026 and is projected to advance at a resilient CAGR of 8.9% from 2026 to 2031, culminating in a forecasted valuation of US$1.10 billion by the end of the period. The Europe pen needles market is propelled by soaring rate of diabetes prevalence, growing numbers of older adults, high levels of obesity, augmented uptake of insulin and GLP-1 pens leading to increased per-patient needle usage. Restraints listed would include challenges associated with pricing pressure, tender-driven commoditization, disparities in reimbursement across countries, and a decline in needle demand due to the longer-acting injectables. Emerging opportunities come through premiumization with safety-engineered and ultra-thin needles, sustainability-focused designs, digital/connected disposables, the expanding e-pharmacy and home-care channels, and OEM collaborations with pen manufacturers to support differentiated products, improve adherence, and protect margins in today's value-driven healthcare environment.
KEY TAKEAWAYS
-
BY REGIONFrance is expected to register the highest CAGR of 9.7%.
-
BY TYPEBy type, the standard pen needles segment dominated the market, with a share of 84.2% in 2025
-
BY LENGTHBy length, the 8mm segment dominated the market, with a share of 31.1% in 2024
-
BY APPLICATIONBy application, insulin therapy will be the fastest-growing segment in the forecast period.
-
BY MODE OF PURCHASEBy mode of purchase, the over-the-counter segment dominated the market, with a share of 30.8% in 2025
-
BY SETTINGBy setting, the home care segment is expected to register the highest CAGR of 9.2%.
-
COMPETITIVE LANDSCAPE - Key PlayersEmbecta Corp., Novo Norisk A/S and B.Braun SE were identified as Star players in the Europe pen needles market, as they have focused on innovation and have broad industry coverage and strong operational and financial strength.
-
COMPETITIVE LANDSCAPE - Start UpsAdvaCare Pharma, MHC Medical Products, and Wellion distinguished themselves among startups and SMEs due to their strong product portfolio and business strategy
Factors such as increasing prevalence of diabetes, the aging population, rising obesity, and the adoption of insulin or GLP-1 pens, which allow for higher needs for needles per patient, further drive the European pen needles market. These, however, are counteracted by major limiting factors such as increased pricing pressure due to tender-based procurement, along with reimbursement inconsistencies across countries, and the reduced needle volume due to longer-acting injectable products. Major opportunities exist in safety-engineered and ultra-thin needle innovation, eco-friendly and connected disposable solutions, the growing e-pharmacy and home-care channels, and OEM partnerships to support premiumization, adherence improvement, and strengthened distribution across Europe's evolving outpatient care landscape.
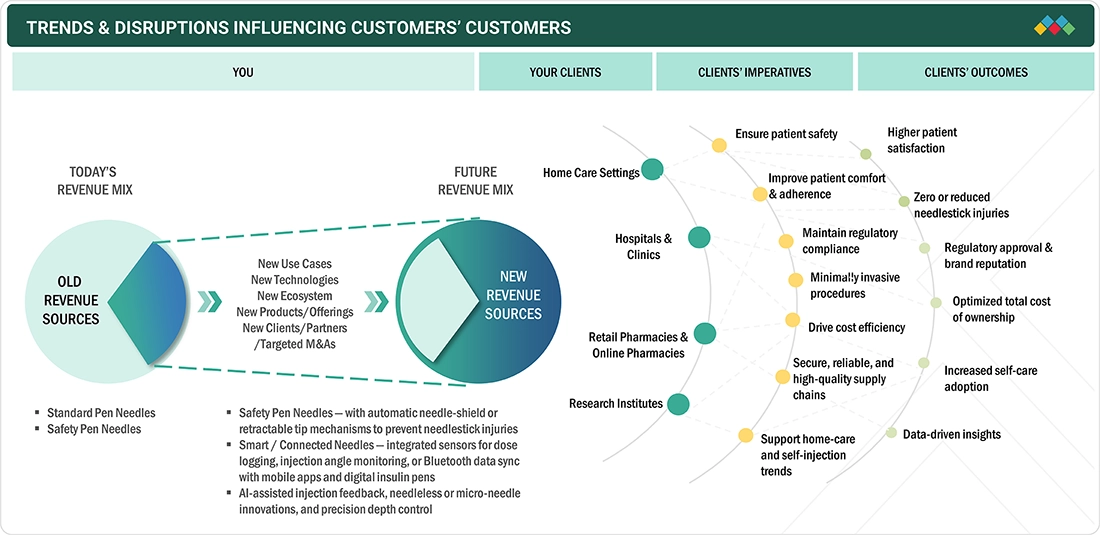
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The gradual improvement of the Europe pen needles market is associated with the increasing incidences of diabetes and the need for pen-based insulin and GLP-1 delivery, which provides additional convenience and accurate dosing. Healthcare providers recommend safety-engineered needles with ultra-thin profiles for maximized patient comfort and minimized needlestick risks, in accordance with the stringent EU safety regulations. The innovation in needle design, coatings, and ergonomics has increased adoption rates, while the growing applications in home care and the rise in digital pharmacy channels affect distribution. On the other hand, increased awareness and skills in diabetes self-management are driving manufacturers to produce diversified, patient-centered pen needle solutions that prioritize comfort, safety, and adherence.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising diabetes prevalence

-
Shift toward home-based care and self-administration of injectable therapies
Level
-
High penetration of low-cost competitors
-
Declining needle consumption due to longer-acting injectables
Level
-
Rising demand for ultra-thin, pain-minimizing, ergonomic pen needles to improve patient comfort
-
OEM partnerships with pen manufacturers for integrated, differentiated delivery solutions.
Level
-
Intense competition among major brands, private labels, and contract manufacturers.
-
Regulatory compliance costs for evolving EU safety and environmental standards.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising diabetes prevalence
An escalating prevalence rate of diabetes, and more specifically an increase in the number of people with insulin-dependent Type 2 diabetes, is a principal driver of the European pen needles market. As more people enter advanced phases of diabetes, requiring insulin injection as a treatment, there will be an augmenting demand for easy-to-use and efficient injection devices. Pen needles have been preferred and increasingly adopted as an alternative offering greater convenience and accuracy compared to conventional injection devices. Moreover, with an ever-aging and expanding patient demographic with diabetes, there will be a heightened favor for shorter and thinner needles, promoting easier and more satisfactory user experiences. The need for safety and satisfaction will drive and sustain market demand, with an exponentially increasing demand stemming from the rising prevalence of diabetes. Moreover, with an intensifying preference among medical practitioners for using insulin pens among patients due to their ease and safeguarding against errors, there will be an exponentially rising dependence on more frequent and repeated needle usage, thus fueling the market.
Restraint: High penetration of low-cost competitors
Low-cost competitors account for the bulk of barriers in the Europe pen needles market since they exert unremitting downward pressure on price, as well as marginalize the great brands. The private label and contract-manufactured pen needles are essentially of inferior quality but are priced significantly lower than those of established brands; thus, they would appeal to payers, group purchasing organizations, and large pharmacy chains. As more reimbursement systems become incentivized toward adopting the lowest-cost option, premium manufacturers must develop increasingly bright arguments why their higher prices should be justified, even when the premium they sell is for comfort, safety features, and brand trust. Thus, this particular scenario limits the commercial success of differentiated products and also retards innovation in terms of speed, as the returns on R&D investment become tighter. In addition, aggressive contracts with low-cost dealers can lock in long-term agreement volumes, which reduces market share mobility for premium dealers. Ultimately, the expanding footprint of value brands turns pen needles into a commoditized category, restricting revenue growth and weakening pricing power for established manufacturers across the European market.
Opportunity: Rising demand for ultra-thin, pain-minimizing, ergonomic pen needles to improve patient comfort
Emerging demands are for ultra-thin, minimal pain, and very ergonomic pen needles, good opportunities in the European market as patient preference is increasingly governing a choice of device and adherence. As more and more people self-administer insulin or GLP-1 injections at home, their demands have grown considerably regarding needles that reduce pain, anxiety, and trauma at the site of injection. Manufacturers that provide such advanced designs, such as tapered tips, multi-bevel needles, silicone lubrication, and flexible hub technologies, stand to differentiate themselves in a market otherwise characterized by commodity products. Innovations around comfort also appeal to newly diagnosed patients, older adults who have dexterity problems, and those who have to take a dose frequently. Healthcare providers will also prefer to recommend premium ergonomic needles once they see improvements in adherence and patient satisfaction; this transformation allows manufacturers to command better pricing and value-based contracts as well as build more robust partnerships with pharmaceutical companies that offer injectable therapies. The entire comfort trend offers opportunities for premiumization and continuous growth.
Challenge: Intense competition among major brands, private labels, and contract manufacturers.
In the European pen needles market, an intense competition between various major companies, private labels, and contract manufacturers poses the pertinent challenge of continuous price pressure, thereby minimizing any window for differentiation. Major players are concerned not only about competing against themselves but also with private-label products sold at a low cost that heavily feature on pharmacy shelves and payer formularies. The situation is exacerbated by the pressure exerted by contract manufacturers that provide high-volume, low-cost products with generic regulatory approval and performance. As a result, pen needles are increasingly seen by the buyers as commodity items, encouraging the whole category toward commoditization; this renders standing ground for premium brands to hold onto its market share and prove that its price should be higher, even with sufficient comfort or safety features. This is further compounded by the aggressive contracting by payers, GPOs, and retail chains, which limits access to certain brands and causes abrupt swings in volume. For manufacturers, competitive pressure drives up customer acquisition costs, reduces margins, and makes long-term investments in innovation and manufacturing upgrades nearly impossible.
europe-pen-needles-market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
As a leader in the industry, these standard and safety pen needles have been well engineered and manufactured to perfection to deliver consistent outcomes with low-pain penetrations, more than decades of clinical validation | Ensures consistent insulin delivery, maximizes patient comfort, and promotes high adherence, preferred by institutions, pharmacies, and home care |
 |
These proprietary PenNeedles are designed for optimal compatibility with Novo pens and constructed with ultra-thin wall technology and smooth-glide coatings | Enhancing comfort, accuracy, and user confidence translates to a seamless and integrated therapy experience across platforms for insulin and GLP-1 pens |
 |
A portfolio of strong and cheap pen needles focusing on safety, sharpness, and minimum insertion force; heavily supported by European manufacturing | Has provided reliable performance to survive competitive tendering conditions, offering safe and comfortable injection solutions in major public healthcare systems |
 |
The company is raising the gold standard in safety, comfort, and performance of pen needles with an advanced mechanical sharpening system, silicone coating, ergonomics, and, above all, patient safety and comfort | Low-pain, accurate injections are the features sought to make daily therapy comfortable, promote compliance in the long run, and act as a cost-effective option for health systems and home-care end-users |
 |
Proprietary sharpening and lubrication techniques were developed apropos of advanced needle technologies, creating a smooth penetration with little pain and a consistent flow. | Increases patient satisfaction, diminishes anxiety regarding injections, and offers accurate and high-quality performance for either delicate injections or larger volumes |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
Before pen needles penetrate healthcare provision and the patients in Europe come from diverse sources such as manufacturing, distribution, research, and technological enhancement. Their manufacturing is adopted as an important standard by the manufacturers who understand the safety pen needles that conform to EU standards of safety, comfort, and precision. Digital health management tools and linked drug delivery platforms increasingly support these products across the spectrum. Given by these public and private health systems are the distributors, procurement agencies for hospitals, and networks of pharmacies. R&D teams, academic collaboratives, and CRO networks in Europe enhance progress in needle geometry, coatings, and safety mechanisms. The end-users of the product are the diabetic patients who primarily use insulin or GLP-1 injectables; they want injections that are painless and reliable and are looking for easy administration. Medical practitioners, diabetes educators, and pharmacists have a significant influence on product selection, particularly when it comes to safety-engineered devices. Ultimately, the input of regulatory authorities, agencies, reimbursements, and professional diabetes societies is significant, establishing set standards for pricing and access policies, thereby influencing the overall market dynamics for pen needles in Europe.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
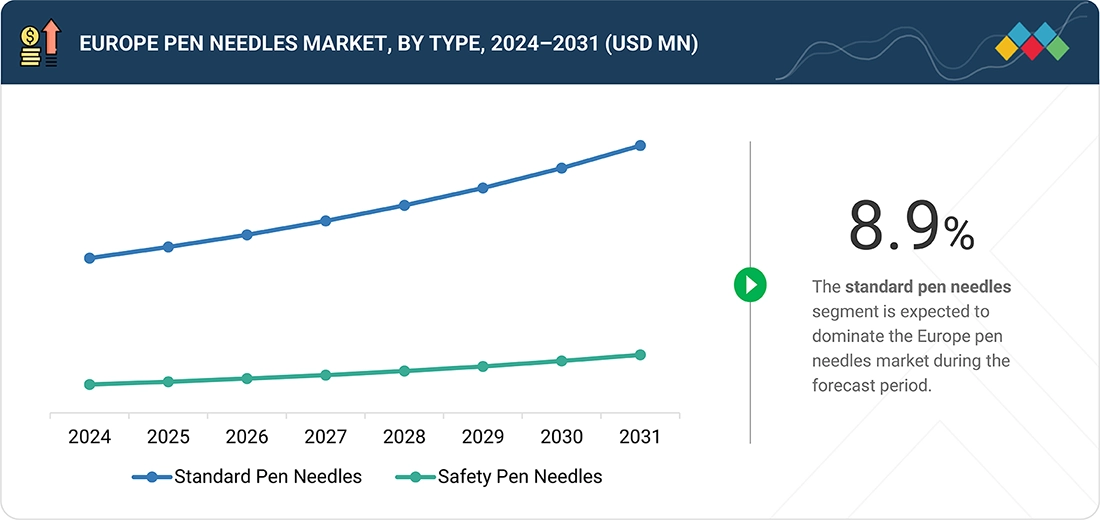
Europe Pen Needles Market, By Type
A high proportion of the Europe pen needle market is dominated by standard pen needles because they mostly have general compatibility and offer credible performance at a much lower price compared to specialty or premium designs. Because they are available from pharmacies, payer-preferred formularies, and GPO contracts, they make them the default choice for the majority of insulin and GLP-1 users. Strong reimbursement support, very low training requirements, and the familiarity of the provider base further consolidate the standard needles' strength. Hence, standard needles are the most accessible and economical choice for populations with a large number of patients.
Europe Pen Needles Market, By Length
8 mm pen needles own the maximum share of the Europe market as they have been the most commonly used type of length in diabetes care for quite some time and are thus very familiar to both providers and patients. Their stronger historical use has built confidence in the clinical setting, particularly for individuals with high BMI who require deeper subcutaneous delivery. Broad reimbursement, availability in numerous pharmacies, and compatibility with most insulin pens have also bolstered the use of 8 mm needles. Consequently, many long-term insulin users would trust 8 mm needles as the default option.
Europe Pen Needles Market, By Application
Insulin therapy commands the largest market share in the Europe pen needles market due to the requirement for patients to administer lifelong injections regularly into their own bodies, creating a steady and large-volume demand scenario for a couple of million diabetes patients on insulin. The bulk of insulin users prefer pen devices to ensure convenience, accurate dosing, and ease of self-administration; hence, their steady consumption of needles. The gradual progression of Type 2 diabetes into insulin-dependent diabetes has also widened the population of users. Well-defined clinical guidelines, robust reimbursement, and no restrictions from major stakeholders strengthen the claim of insulin therapy being the leading driving force for pen needle usage in the Europe market.
Europe Pen Needles Market, By End User
Home care is the largest arena of pen needle business in Europe because most diabetic patients self-administer their OW insulin or GLP-1 injections outside of the clinical setting, thus creating a substantial volume for convenient and easy-to-use pen needles. In-home management decreases healthcare visits, aligns with payer cost-containment interests, and promotes patient independence. Pharmacies and mail-order services facilitate wide access, and simple training makes self-injection practical across age groups. Therefore, home care will remain the primary use of the Europe pen needle market.
Europe Pen Needles Market, By Mode of Purchase
In the Europe pen needles market, over-the-counter (OTC) sales account for the bulk of sales because they allow daily, immediate, and cash purchasing away from the drug prescriptive requirements that hamper large classes of patients trying to manage their injections at home. Standard pen needles in pharmacies and retail chains are stocked in a great variety and at relatively low cost. At the time of retailing, such prices often reflect the payer's or pharmacy benefit manager's proclivities. The fact that OTC products avoid many administrative hurdles of refill means that patient compliance can be enhanced. Thus, the combination of being easily and economically available endows OTC outlets with the lion's share of the distribution in the European pen needles market.
REGION
France to be fastest-growing region in europe pen needles market during forecast period
France is expected to be the fastest-growing country in the Europe pen needles market during the forecast period due to a combination of demographic, regulatory, and healthcare system factors. Rising diabetes prevalence and an aging population are increasing demand for insulin and GLP-1 injectable therapies. Strong reimbursement coverage for diabetes care improves patient access to pen needles and supports consistent usage. France is also witnessing rapid growth in home-care treatment and e-pharmacy adoption, making pen needles more accessible. Additionally, government emphasis on patient safety and adherence is accelerating the uptake of safety-engineered and premium pen needles, driving faster market expansion.
europe-pen-needles-market: COMPANY EVALUATION MATRIX
In the Europe pen needles market, Embecta Corp. (Star) has a strong and established product portfolio and a vast geographic presence. Owen Mumford (Emerging Leader) has substantial product innovations compared to its competitors. While they have broad product portfolios, they lack a strong growth strategy for business development.
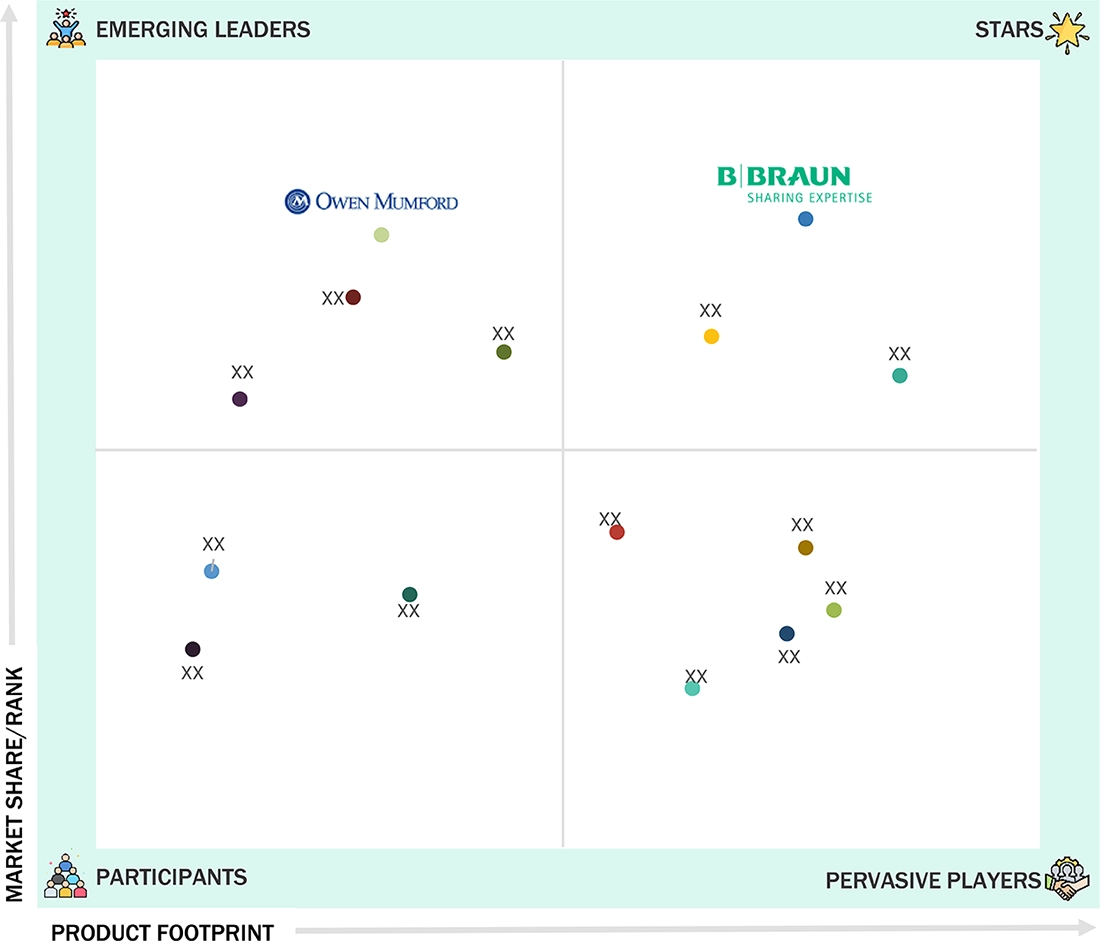
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Embecta Corp. (US)
- Novo Nordisk A/S (Denmark)
- MTD Medical Technologies and Devices (Italy)
- B. Braun SE (Germany)
- Terumo Corporation (Japan)
- Owen Mumford (UK)
- NIPRO Corporation (Japan)
- Allison Medical, Inc. (US)
- AdvaCare Pharma (US)
- Berpu Medical Technology Co., Ltd. (China)
- ARKRAY, Inc. (Japan)
- GlucoRx Limited (UK)
- HTL-STREFA (Poland)
- UltiMed, Inc. (US)
- MHC Medical Products, LLC (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size Value in 2026 (Value) | USD 0.72 BN |
| Revenue Forecast in 2031 (Value) | USD 1.10 BN |
| Growth Rate | 8.90% |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD MN), Volume (Thousand Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered | By Type: Standard Pen Needles, Safety Pen Needles, By Length: 8mm, 5mm, 6mm, 4mm, 12mm, 10mm, By Application: Insulin Therapy, GLP-1 Therapy, Growth Hormone Therapy, Osteoporosis, Other Applications, By Mode of Purchase: Prescription based, Over the Counter (OTC), Online Purchase, Other Mode of Purchases, By End User: Home Care, Hospitals and Clinics and Other End User |
| Country Covered | Germany, France, UK, Spain, Italy and Rest of Europe |
| Parent & Related Segment Reports |
Pen Needles Market US Pen Needles Market |
WHAT IS IN IT FOR YOU: europe-pen-needles-market REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Comparison of pen needle types by length (4mm, 5mm, 6mm, 8mm, and 12mm), needle gauge, and compatibility with various insulin and GLP-1 pens. | Assessment of technological innovations such as ultra-thin wall designs, safety-engineered needles, and low-friction coatings to enhance injection comfort and precision. |
| Company Information | Profiles of key manufacturers such as BD (US), Novo Nordisk (Denmark), B. Braun (Germany), Terumo (Japan), and Owen Mumford (UK). | Market share benchmarking and competitive landscape of leading players across North America, Europe, Asia Pacific, and the Middle East. |
| Geographic Analysis | Detailed regional assessment of North America, Europe, Asia Pacific, Latin America, and Middle East & Africa markets. | Country-level sizing, CAGR forecasts, and adoption analysis for major markets such as the US, Germany, Japan, China, and India. |
RECENT DEVELOPMENTS
- January 2023 : Embecta Corp. (US) expanded its presence in New Jersey with the opening of a new global headquarters in Parsippany.
- March 2023 : Novo Nordisk A/S (Denmark) The company aims to expand its presence in the US, near the greater Boston metro area, creating one of the largest R&D hubs outside Denmark.
- March 2024 : MTD (Medical Technology and Devices) (Italy) acquired Ypsomed’s pen needle and blood glucose monitoring (BGM) businesses. This strategic move will solidify MTD’s position as a globally leading player in pen needle production and will enhance its comprehensive solution portfolio of diabetes care.
- Nvember 2022 : Embecta Corp. (US) and Intuity Medical, Inc. (US) both companies signed a co-promotional agreement under which sales representatives of Embecta will promote Intuity Medical’s innovative POGO Automatic Blood Glucose Monitoring System to healthcare professionals within the US.
Table of Contents

Methodology
This research study involved the extensive use of both primary and secondary sources. It involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, the competitive landscape of market players, and key market dynamics such as drivers, opportunities, challenges, restraints, and key player strategies.
Secondary Research
This research study involved the wide use of secondary sources, directories, databases such as Dun & Bradstreet, Bloomberg Businessweek, and Factiva, white papers, and companies’ house documents. Secondary research was undertaken to identify and collect information for this extensive, technical, market-oriented, and commercial study of the Europe Pen Needles Market. It was also used to obtain important information about the top players, market classification, and segmentation according to industry trends to the bottom-most level, geographic markets, and key developments related to the market. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various supply side and demand side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, engineers, and related key executives from various companies and organizations operating in the Europe Pen Needles Market. Primary sources from the demand side included hospitals, clinics, researchers, lab technicians, purchase managers etc, and stakeholders in corporate & government bodies.
Market Size Estimation
The market size for Europe Pen Needles Market was calculated using data from four different sources, as will be discussed below. Each technique concluded and a weighted average of the four ways was calculated based on the number of assumptions each approach made. The market size for Europe Pen Needles Market was calculated using data from three distinct sources, as will be discussed below:
Data Triangulation
The entire market was split up into five segments when the market size was determined. Data triangulation and market breakdown processes were used where necessary to complete the entire market engineering process and arrive at the precise statistics for all segments.
Approach to derive the market size and estimate market growth.
Using secondary data from both paid and unpaid sources, the market rankings for the major players were determined following a thorough analysis of their sales of pen needles. Due to data restrictions, the revenue share in certain cases was determined after a thorough analysis of the product portfolio of big corporations and their individual sales performance. This information was verified at each stage by in-depth interviews with professionals in the field.
Market Definition
A pen needle is a hollow needle entrenched in a plastic hub and attached to injectable pens. Pen needles are available in a wide range of lengths and diameters and can be used by healthcare professionals and patients to deliver injectable medications into the patient’s body. Pen needles are electro-polished for smooth, thin, and fine-point tips and provide comfort and ease in penetration; they serve as an alternative drug delivery technique to the traditional method of injecting drugs.
Stakeholders
- Manufacturers of pen needles, insulin pens, injectable drug delivery devices, and diabetes supplies
- Original equipment manufacturing companies
- Suppliers and distributors of pen needles, insulin pens, injectable drug delivery devices, and diabetes supplies
- Healthcare service providers
- Teaching hospitals and academic medical centers
- Health insurance players
- Government bodies/municipal corporations
- Regulatory bodies
- Business research and consulting service providers
- Authorities framing reimbursement policies for pen needles
- Venture capitalists
- Market research and consulting firms
Report Objectives
- To define, describe, and forecast the Europe Pen Needles Market based on type, length, application, mode of purchase, setting and region.
- To provide detailed information about the major factors influencing the market growth (such as drivers, restraints, challenges, and opportunities)
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall Europe Pen Needles Market
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for key players
- To profile the key players and comprehensively analyze their market shares and core competencies in the Europe Pen Needles Market
- To track and analyze competitive developments such as partnerships, expansions, acquisitions, collaborations, product launches, agreements, and other developments in the Europe Pen Needles Market
- To benchmark players within the Europe Pen Needles Market using the Company Evaluation Quadrant framework, which analyzes market players on various parameters within the broad categories of business strategy, market share, and product offerings.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Pen Needles Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Europe Pen Needles Market