
Asia Pacific eClinical Solutions Market Size, Growth, Share & Trends Analysis
Asia Pacific eClinical Solutions Market by Product (CDMS, EDC, CTMS, eCOA, RTSM, eTMF, RIMS, eConsent), Application (Collection, Operation, Analytics), Trial Phase, End User (Pharma, Biotech, Medtech), Trends, Growth, Market Insight - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
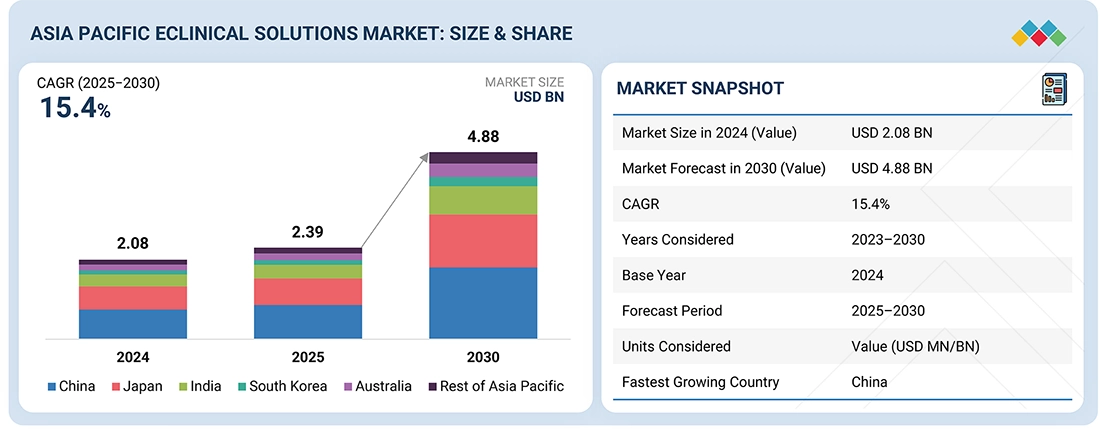
The Asia Pacific eClinical solutions market, valued at US$2.08 billion in 2024, stood at US$2.39 billion in 2025 and is projected to advance at a resilient CAGR of 15.4% from 2024 to 2030, culminating in a forecasted valuation of US$4.88 billion by the end of the period. The Asia Pacific eClinical solutions market is experiencing rapid growth, driven by increasing clinical trial activity, expanding digital infrastructure, and the rising adoption of advanced technologies across the region’s research ecosystem.
KEY TAKEAWAYS
-
By CountryThe eClinical solutions market in China is expected to register the highest CAGR of 15.9%.
-
By ProductBy product, the Electronic Data Capture & Clinical Data Management Solutions segment registers the largest share of 20.7% in 2024.
-
By Deployment ModelBy deployment model, the web-based & cloud-based models segment registered the largest share of 89.8% in 2024.
-
By ApplicationsBy application, in 2024, the data collection accounted for the largest share in the Asia Pacific eClinical solutions market.
-
By Clinical Trial PhaseBy clinical trial phase, the Phase IV trial segment is expected to grow the fastest during the forecast period.
-
By End userBy end user, the pharmaceutical & biopharmaceutical companies accounted for the largest share in the Asia Pacific eClinical solutions market in 2024.
-
Competitive LandscapeMedidata, Veeva Systems, and ICON plc were identified as some of the star players in the Asia Pacific eClinical solutions market, given their strong market share and product footprint.
-
Competitive LandscapeCastor and Medrio, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The eClinical solutions market in Asia Pacific is expanding rapidly as the region strengthens its position as a global clinical trial hub, supported by large patient populations and increasing multinational study activity that demands more efficient digital workflow. Increasing adoption of patient-centric technologies such as ePRO, eConsent, and remote monitoring is enhancing trial efficiency and data quality.
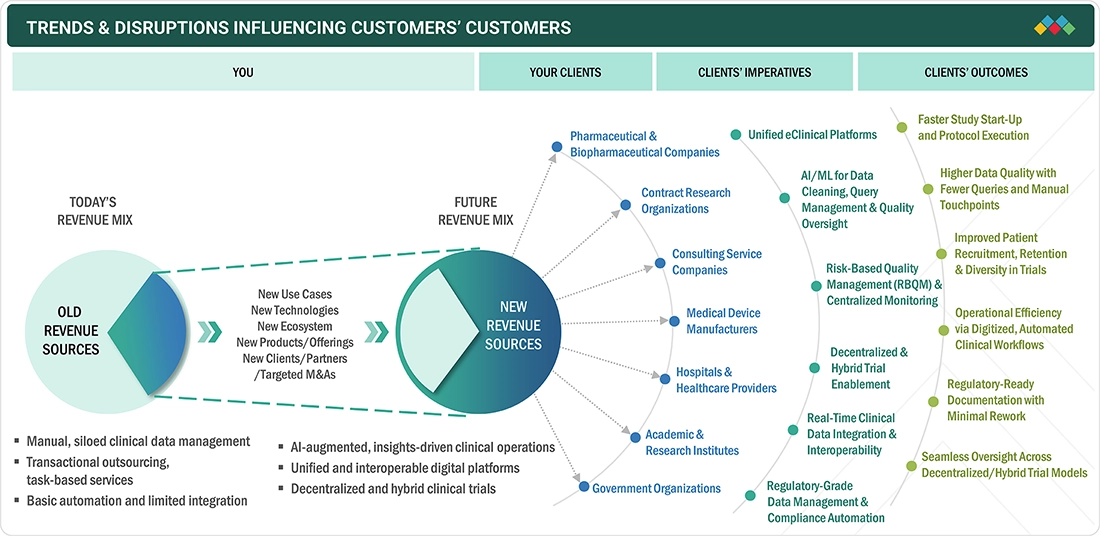
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
Rapid digital adoption is changing the Asia Pacific eClinical solutions market. To enable multi-country trial operations, sponsors and contract research organizations are increasingly adopting unified eClinical platforms, AI-driven data quality tools, and cloud-based architectures. More effective decentralized and hybrid trial models are made possible by the increasing usage of eConsent, eCOA, remote monitoring, and risk-based monitoring. The region's shift to contemporary, technologically enabled clinical research is being accelerated by increased expectations for real-time data integration, regulatory-grade documentation, and better patient recruitment and retention.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Expanding global clinical trial hub

-
Government support for digital trial adoption
Level
-
High technology and deployment costs
-
Shortage of skilled clinical data talent
Level
-
Growing outsourcing of clinical trials
-
Increasing shift to decentralized trials
Level
-
Complex data protection and compliance requirements
-
Infrastructure gaps between developed and developing countries
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Expanding global clinical trial hub
The rising stature of Asia Pacific as an global hub for conducting clinical trials has a direct association with the rising demand for eClinical solutions within the region. Clinical trials are attracted to counties with large populations, streamlined recruitment procedures, low costs, and flexible regulatory standards. Countries that are poised with these opportunities are China, India, South Korea, and Australia. EDC, eCOA, CTMS, and eConsent solutions are increasingly adopted within the region as more and more trials enter Asia Pacific. In addition, efforts at national levels within Asia Pacific nations, such as national health tech projects within Singapore, are pushing the transition towards modern and data-driven clinical trial solutions.
Restraint: High technology and deployment costs
The Asia Pacific eClinical solutions industry is still significantly hampered by high technology and deployment costs, particularly in nations with poor digital infrastructure and tight research budgets. A substantial investment in software licensing, system integration, data protection, and training is necessary for the implementation of sophisticated systems like EDC, CTMS, eCOA, and remote monitoring tools. The adoption of digital trial technology can be slowed by these costs, which can be difficult for smaller CROs, hospitals, and regional pharmaceutical corporations in Asia Pacific. Furthermore, it is challenging to scale eClinical solutions uniformly throughout the Asia Pacific area due to the considerable diversity in technical preparedness among the region's nations, which raises deployment complexity and costs.
Opportunity: Growing outsourcing of clinical trials
Strong opportunities are being created for local eClinical solution providers by the growing trend of outsourcing clinical trials to Asia Pacific, which is becoming more and more significant in the global drug development process. APAC's sizable and varied patient populations, cost advantages, highly qualified workforce, and more accommodating regulatory regimes in nations like China, India, and South Korea attract pharmaceutical businesses. Sponsors are searching for scalable digital solutions that can streamline intricate, multi-site studies as clinical trial activity increases. The need for tools like randomization technologies, clinical trial management systems (CTMS), and electronic data capture (EDC) is being driven by this. The APAC eClinical solutions market is anticipated to expand quickly as more trials are contracted out to contract research organizations (CROs) as the area continues to transition to cloud-based operations.
Challenge: Complex data protection and compliance requirements
The Asia Pacific eClinical solutions market is challenged by complex data protection and compliance standards, where strict safeguards for patient data in clinical trials are required due to disparate regulations in China, India, Japan, and Australia. In addition to local laws like China's Personal Information Protection Law (PIPL) and India's Digital Personal Data Protection Act (DPDPA), which enforce data localization, consent, and breach reporting, providers must comply with international standards like EU GDPR and US FDA 21 CFR Part 11 as platforms like electronic data capture (EDC) and clinical trial management systems (CTMS) expand due to outsourcing. In addition to raising the possibility of fines, harm to one's reputation, and delays from cloud-based cybersecurity concerns, this increases expenses through customized encryption, audits, and interoperability.
ASIA PACIFIC ECLINICAL SOLUTIONS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Providing Asia Pacific eClinical platforms such as EDC, eConsent, RTSM, and DCT-enabling tools that support multi-country studies across China, Japan, South Korea, India, and Australia, while aligning with local regulatory frameworks (e.g., PMDA, TGA, CDSCO, NMPA) | Enables smoother multi-country trial execution| Accelerates study start-up in diverse regulatory environments| Ensures high-quality, compliant data handling across mature and emerging research markets |
 |
Deploying its unified clinical operations suite including CTMS, eTMF to support biopharma, CROs, and research hospitals conducting regional and global trials involving Asia Pacific sites | Improves visibility and oversight of Asia Pacific studies | Speeds site activation across multiple countries| Reduces operational inefficiencies in complex regional trials with varied regulatory timelines |
 |
Offering technology-enabled contract research organization services using eCOA, eSource, remote monitoring, and advanced analytics tailored to Asia Pacific markets, with localization for languages, regulatory requirements, and data-privacy frameworks (e.g., PDPA, PIPL) | Ensures compliant and secure data capture| Enhances quality of patient-reported and site-entered+C2 data across culturally diverse populations| Supports scalable execution of large, multi-country clinical programs |
|
|
Implementing hybrid and decentralized trial models using digital monitoring tools, virtual visit solutions, digital recruitment, and site-support platforms designed for Asia Pacific's geographically dispersed and diverse patient populations | Expands patient access across remote and urban areas| Reduces site burden| Enhances recruitment and retention| Supports efficient trial execution in regions with varied healthcare infrastructure |
 |
Delivering validated eCOA, eConsent, and decentralized trial solutions tailored for Asia Pacific regulatory needs, with multilingual capabilities for markets including China, Japan, Korea, and India | Provides consistent patient and site experience across Asia Pacific countries| Improves accuracy of clinical outcome data| Streamlines consent and engagement in large, culturally diverse studies |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The ecosystem includes significant pharmaceutical and research end customers, startups, established technology providers, and cloud infrastructure partners. The region's shift to digitized, linked, and efficient clinical trials is accelerated by this stakeholder convergence. It is an ecosystem that encourages the increasing use of decentralized trials, cloud-based deployment models, and platform unification, all of which improve the general digital maturity of clinical research in Asia.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Asia Pacific eClinical Solutions Market, By Product
In 2024, the electronic data capture & clinical data management solutions segment held the largest share of the Asia Pacific eClinical solutions market. The segment is driven by clinical research activity in the area, with countries such as South Korea, China, India, and Australia continuing to draw an increasing number of local and international trials. To manage extensive patient recruitment, improve data integrity, and simplify the complexities of multi-country trial operations, sponsors and research sites in the Asia Pacific are increasingly relying on EDC and CDMS platforms. The transition to digital data management is being accelerated by growing investments in cloud-based platforms, the increasing adoption of decentralized and hybrid trial models, and the tightening of data privacy laws throughout the region.
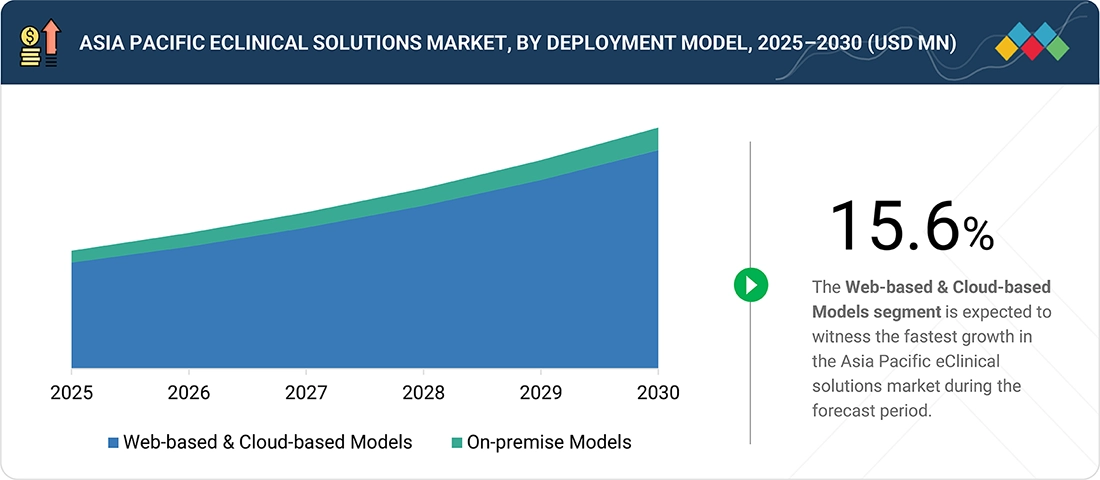
Asia Pacific eClinical Solutions Market, By Deployment Model
In 2024, the web-hosted and cloud-based deployment segment held the largest share of the Asia Pacific eClinical solutions market. The region's growing investment in digital health infrastructure and the need to support extensive and varied clinical trial networks are the driving forces behind this. Since cloud solutions can manage large patient loads, facilitate remote data collection, and ensure consistent performance across geographically dispersed sites, research companies throughout Asia are giving them top priority. Cloud-based systems are especially appealing in markets where technology adoption varies significantly between urban and rural research environments, due to their scalability and reduced maintenance requirements.
Asia Pacific eClinical Solutions Market, By Application
In 2024, the data collection segment accounted for the largest share of the Asia Pacific eClinical solutions market. The region's rapidly developing clinical research activity and the increasing demand for precise, up-to-date patient data in various trial settings are the primary causes of this. As digital tools like electronic data capture (EDC), eCOA, ePRO, and eSource become more widely used, sponsors and CROs in the Asia Pacific are giving priority to technology that facilitates large-scale, multi-country studies, improves data quality, and expedites data entry. The demand for sophisticated data collection technologies that can combine site-based and remote inputs is further accelerated by the region's transition to decentralized and hybrid trial models.
Asia Pacific eClinical Solutions Market, By Clinical trial phase
In 2024, Phase III held the largest share of the Asia Pacific eClinical solutions market, demonstrating the area's capacity to serve sizable patient populations and its expanding involvement in late-stage drug development. Strong digital platforms are becoming increasingly necessary to handle complex trial procedures as international pharmaceutical corporations conduct Phase III investigations in countries such as China, India, Japan, South Korea, and Australia. The use of cutting-edge eClinical solutions, such as EDC, CTMS, eCOA, and remote monitoring systems, is driven by the need for substantial data gathering, strict monitoring, and strong regulatory compliance in these late-stage trials. Phase III studies are the most significant contributor to the use of eClinical solutions in the Asia Pacific area due to their size and complexity, which makes digital enablement crucial.
Asia Pacific eClinical Solutions Market, By end user
In 2024, pharmaceutical and biopharmaceutical companies accounted for the largest share of the Asia Pacific eClinical solutions market, driven by their expanding R&D activities and increasing involvement in local, regional, and global clinical trials. Pharmaceutical companies are adopting cutting-edge eClinical solutions to enhance data accuracy, accelerate trial activities, and enable decentralized and hybrid trials as Countries such as China, India, South Korea, Japan, and Australia accelerate their innovation cycles. eClinical solutions play a vital role in boosting productivity and regulatory compliance, as these enterprises must manage complex, multi-stage research activities. As a result, it can be seen that drug and biotech enterprises remain the principal market drivers and adopters of eClinical solutions within the Asia Pacific region.
REGION
China is expected to be fastest-growing country in Asia Pacific eClinical Solutions market during forecast period
China represents the most rapidly expanding market within the Asia Pacific eClinical solutions market. The impending replacement of traditional methods with more modern technologies represents opportunities and challenges posed and met by China. Moreover, due to China’s extensive patient base and acceleration in trial approval, investment from global and local pharmaceutical giants contributes highly to adopting cutting-edge technologies within eClinical solutions. China’s focus on standardizing data, electronic documentation, and assistance with decentralized trials drives adoption and usage of EDC, CTMS, eCOA, and eConsent solutions.
ASIA PACIFIC ECLINICAL SOLUTIONS MARKET: COMPANY EVALUATION MATRIX
In the Asia Pacific eClinical solutions market matrix, Medidata (Star) leads with a dominant presence, supported by its comprehensive eClinical platform, integration across EDC, eCOA, RTSM, and strong regional presence, and deep partnerships with leading pharma, biotech, and contract research organizations across the region. EvidentIQ (Emerging Leader) is driven by its focus on advanced analytics, integrated digital platforms, and innovative tools that support modern, data-driven clinical trial execution.
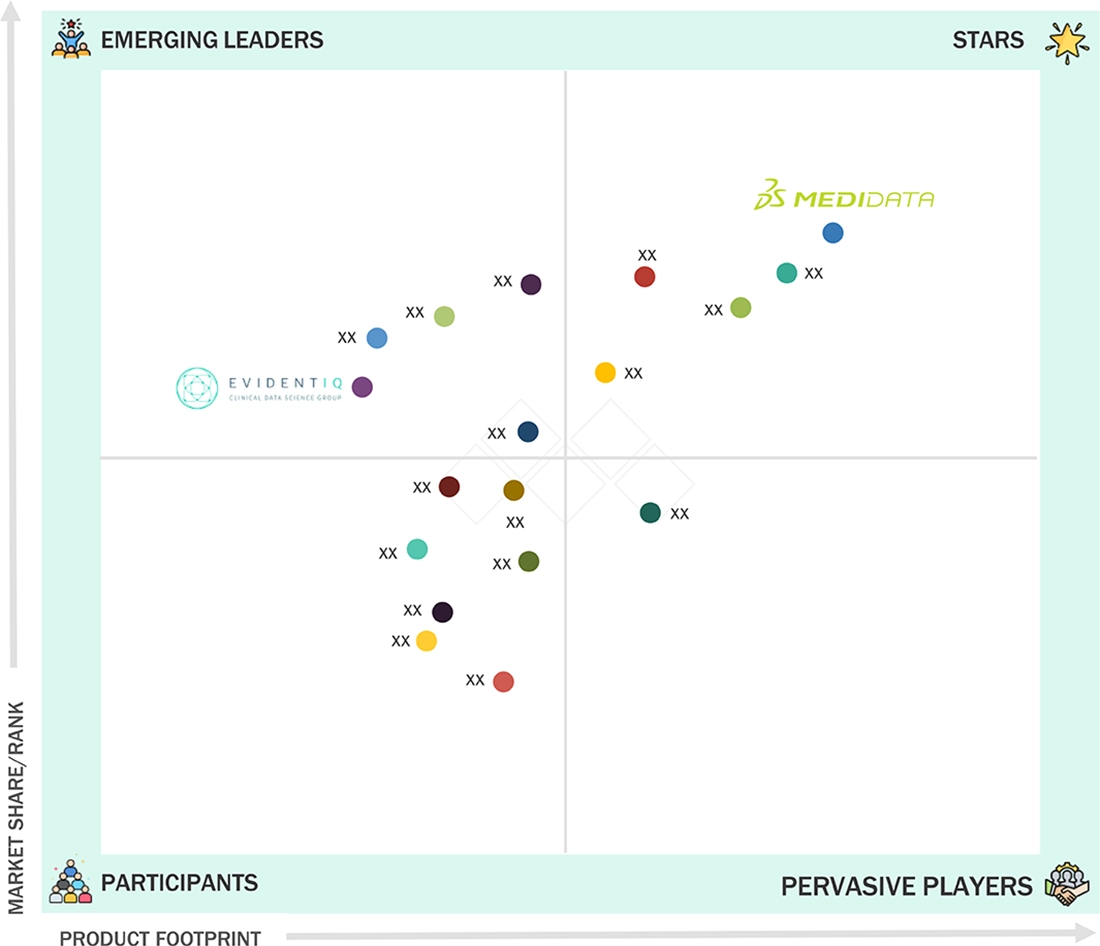
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Medidata (a Dassault Systèmes Company) (France)
- Veeva Systems (US)
- IQVIA (US)
- ICON Plc (Ireland)
- Signant Health (US)
- Oracle (US)
- Clario (US)
- eClinical Solutions LLC (US)
- Clinion (US)
- Maxis IT (US)
- 4G Clinical (US)
- Saama (US)
- Fountayn (US)
- CRSCUBE INC (South Korea)
- Advarra (US)
- Caidya (US)
- OpenClinica, LLC (US)
- EvidentlQ (Germany)
- ArisGlobal (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.08 Billion |
| Market Forecast in 2030 (Value) | USD 4.88 Billion |
| Growth Rate | CAGR of 15.4% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Japan, China, India, Australia, South Korea, and Rest of Asia Pacific |
| Parent & Related Segment Reports |
eClinical Solutions Market US eClinical Solutions Market Europe eClinical Solutions Market |
WHAT IS IN IT FOR YOU: ASIA PACIFIC ECLINICAL SOLUTIONS MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Need for APAC-specific market sizing and growth outlook | Delivered Asia Pacific market sizing, landscape, and 5-year growth potential analysis based on clinical trial activity and digital adoption | Enabled data-driven revenue forecasting and region-specific investment prioritization |
| Requirement to understand APAC regulatory and compliance complexity | Analyzed country-level regulatory bodies, ethics committee processes, and data privacy requirements affecting eConsent adoption |
|
| Identify key growth drivers and opportunities in APAC | Assessed drivers such as increasing clinical trials, decentralized/hybrid trial adoption, and CRO outsourcing across Asia Pacific | Helped identify high-growth countries and priority use cases for expansion |
| Need to understand key restraints and challenges in APAC | Evaluated challenges, including regulatory fragmentation, variable site digital maturity, and data residency concerns | Supported mitigation planning, product localization, and go-to-market customization |
| Request for competitive and supplier landscape assessment | Mapped global and regional eConsent vendors with Asia Pacific presence, including capability benchmarking and adoption levels | Enabled clearer vendor positioning, partnership targeting, and competitive differentiation |
| Recent market and supplier trends impacting APAC | Captured key Asia Pacific-relevant trends such as partnerships with local CROs, regional expansions, hybrid trial enablement, and technology enhancements | Improved strategic awareness of market momentum and competitive moves |
RECENT DEVELOPMENTS
- January 2025 : Medidata (A Dassault Systèmes Company) (France) has launched Clinical Data Studio, an AI-powered software platform designed to modernize clinical trial data management.
- October 2024 : Medidata (A Dassault Systèmes Company) (France) launched Rave Lite, which simplifies data collection and management, enabling research teams to conduct trials efficiently while ensuring compliance with regulatory standards
- September 2023 : ICON plc (Ireland) launched Clinical Trial Tokenization to enhance clinical trial data management by securely linking patient data across different sources while maintaining privacy.
- COLUMN 'A' SHOULD BE IN TEXT FORMAT AND NOT DATE FORMAT :
Table of Contents

Methodology
This research study involved the extensive use of both primary and secondary sources. It involved the analysis of various factors affecting the industry to identify the segmentation types, industry trends, key players, the competitive landscape of market players, and key market dynamics such as drivers, opportunities, challenges, restraints, and key player strategies.
Secondary Research
This research study extensively utilized secondary sources, including directories, databases such as Dun & Bradstreet, Bloomberg Businessweek, and Factiva, as well as white papers, annual reports, and companies' house documents. The aim of the secondary research was to gather and analyze information for a comprehensive and commercially focused study of the Asia Pacific eClinical Solutions Market, encompassing technical aspects and market dynamics. It also facilitated the identification of key players, market classification, industry trends, geographical markets, and significant market-related developments. Additionally, a database of prominent industry leaders was compiled through secondary research.
Primary Research
In the primary research process, various supply-side and demand-side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, engineers, and related key executives from various companies and organizations operating in the Asia Pacific eClinical Solutions Market. Primary sources from the demand side included personnel from pharmaceutical & biotechnology companies, government organizations, research institutes and hospitals (small, medium-sized, and large hospitals).
Market Size Estimation
The total size of the Asia Pacific eClinical Solutions Market was determined after data triangulation through the two approaches mentioned below. After the completion of each approach, the weighted average of these approaches was taken based on the level of assumptions used in each approach.
Data Triangulation
The size of the Asia Pacific eClinical Solutions Market was estimated through segmental extrapolation using the bottom-up approach. The methodology used is as given below: -
- Revenues for individual companies were gathered from public sources and databases.
- Shares of leading players in the Asia Pacific eClinical Solutions Market were gathered from secondary sources to the extent available. In certain cases, shares of eClinical solutions businesses have been ascertained after a detailed analysis of various parameters including product portfolios, market positioning, selling price, and geographic reach & strength.
- Individual shares or revenue estimates were validated through interviews with experts.
- The total revenue in the Asia Pacific eClinical Solutions Market was determined by extrapolating the market share data of major companies.
Market Definition
eclinical solutions are the software/platform that changes the paper-based clinical research model into an electronic form. Such technologies help the researcher in facilitating the process of data collection, its transmission, and surveillance of the clinical trial process and provide enhanced options for better planning and execution of a clinical trial. eClinical technologies fast-track the study by reducing the risk and maximizing resources.
Key Stakeholders
- Healthcare IT Service Providers
- eClinical Solution Vendors
- Clinical Research Organizations
- Pharmaceutical/Biopharmaceutical Companies
- Research and Development (R&D) Companies
- Business Research and Consulting Service Providers
- Medical Research Laboratories
- Government agencies
- Healthcare startups, consultants, and regulators
- Academic Medical Centers/Universities/Hospitals
Objectives of the Study
- To define, describe, and forecast the Asia Pacific eClinical Solutions Market based on product, deployment model, application, clinical trial phase, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall Asia Pacific eClinical Solutions Market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa.
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments such as product launches & approvals, partnerships, agreements, and collaborations in the overall Asia Pacific eClinical Solutions Market
- To benchmark players within the market using the proprietary "Competitive Leadership Mapping" framework, which analyzes market players on various parameters within the broad categories of business and product strategy.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Geographic Analysis
- Further breakdown of the Rest of Asia Pacific Asia Pacific eClinical Solutions Market into Australia, Taiwan, New Zealand, Thailand, Singapore, Malaysia, and other countries
- Further breakdown of the Rest of Europe Asia Pacific eClinical Solutions Market into Russia, Austria, Finland, Sweden, Turkey, Norway, Poland, Portugal, Romania, Denmark, and other countries
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific eClinical Solutions Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Asia Pacific eClinical Solutions Market