
Europe eClinical Solutions Market Size, Growth, Share & Trends Analysis
Europe eClinical Solutions Market by Product (CDMS, EDC, CTMS, eCOA, RTSM, eTMF, RIMS, eConsent), Application (Collection, Operation, Analytics), Trial Phase, End User (Pharma, Biotech, Medtech), Opportunities, Trends, Market Share - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
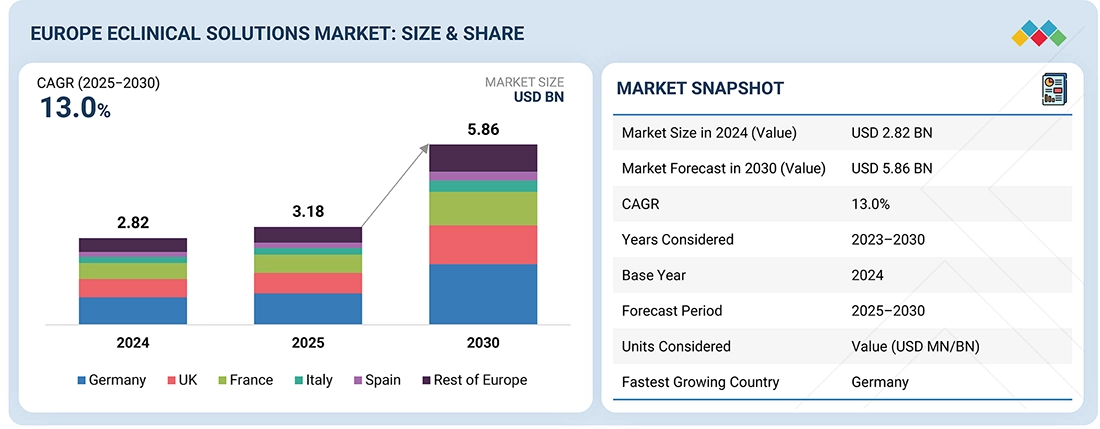
The Europe eClinical solutions market, valued at US$2.82 billion in 2024, stood at US$3.18 billion in 2025 and is projected to advance at a resilient CAGR of 13.0% from 2025 to 2030, culminating in a forecasted valuation of US$5.86 billion by the end of the period. This growth is driven by the increasing shift toward digital and decentralized clinical trial models enabled by CTR and CTIS adoption.
KEY TAKEAWAYS
-
By CountryIn 2024, Germany accounted for a 31.7% revenue share of the Europe eClinical solutions market.
-
By ProductBy product, the Electronic Clinical Outcome Assessment Solutions segment is expected to register the highest CAGR of 15.3%.
-
By Deployment ModelBy deployment model, the web-based & cloud-based models segment is projected to grow at the fastest rate from 2025 to 2030.
-
By ApplicationsBy application, In 2024, the data collection accounted for the largest share (38.3%) in the Europe eClinical solution market.
-
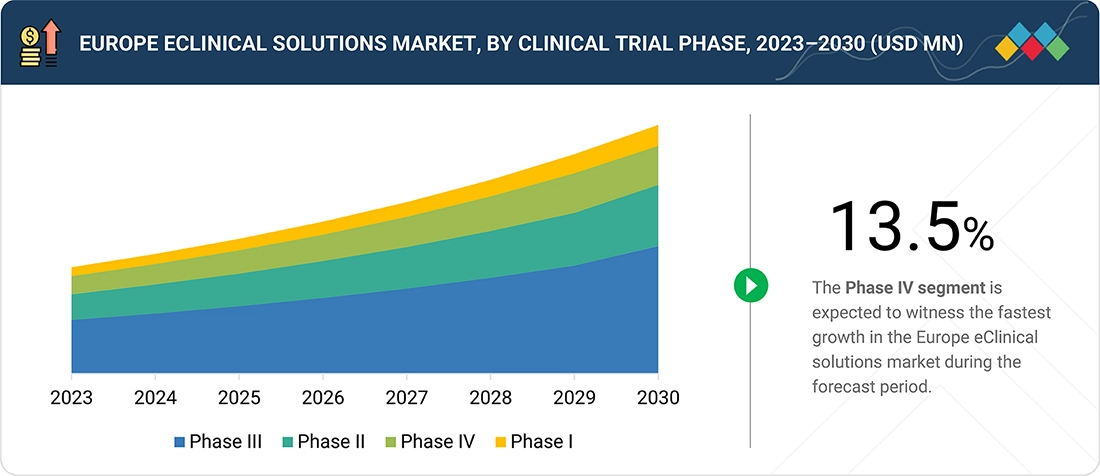
By Clinical Trial PhaseBy clinical trial phase, the Phase IV segment is expected to grow the fastest during the forecast period.
-
By End userBy end user, in 2024, the pharmaceutical & biopharmaceutical companies accounted for the largest share in the Europe eClinical solutions market.
-
Competitive LandscapeMedidata, Veeva Systems, and ICON plc were identified as some of the star players in the Europe eClinical solutions market, given their strong market share and product footprint.
-
Competitive LandscapeOwkin, Inc. and Medrio, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
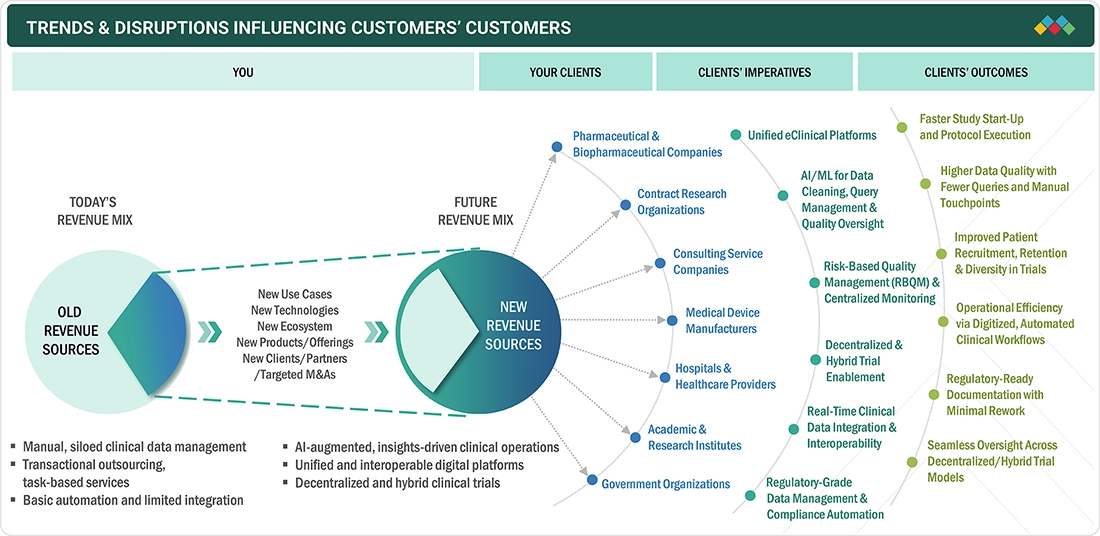
Digital transformation initiatives across European sponsors and research sites are accelerating the shift toward modern eClinical platforms, as the increasing adoption of patient-centric technologies, such as ePRO, eConsent, and remote monitoring, enhances trial efficiency and data quality. Rising demand for streamlined, real-time study operations is further driving investment in scalable eClinical solutions across the region.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The eClinical solutions market in Europe is being reshaped by the mandatory shift to the EU CTR and CTIS, forcing sponsors to redesign their digital trial infrastructure for unified submissions and transparency. The rise of pan-EU data interoperability initiatives is disrupting traditional, siloed EDC setups and pushing vendors to offer more connected platforms. The growing adoption of digital endpoints, validated through European research networks, is expanding the demand for sensor integration and advanced analytics. Meanwhile, the expansion of multi-country hybrid trials is accelerating the move toward patient-facing digital tools that work seamlessly across diverse healthcare systems. These shifts are creating a competitive edge for vendors that can offer compliance-ready, multilingual, and highly modular eClinical ecosystems.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Europe regulatory harmonization boosts need for unified eClinical systems

-
Research funding accelerates digital trial infrastructure demand
Level
-
Strict General Data Protection Regulation (GDPR) rules raise compliance and deployment costs
-
Difficulty in seamless integration of eClinical platforms
Level
-
Growth of RWD/RWE networks creates analytics opportunities
-
Increased adoption of eSource to reduce site workload
Level
-
Heavy validation requirements limit rapid vendor deployment
-
Cross-border data transfer rules complicate architecture choices
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Europe regulatory harmonization boosts need for unified eClinical systems
The implementation of the EU Clinical Trials Regulation and the centralized Clinical Trials Information System (CTIS) platform is prompting sponsors to streamline their management of multi-country studies. As regulatory expectations become more standardized across Member States, the need for interoperable and end-to-end eClinical systems has increased significantly. Unified platforms that support consistent documentation, reporting, and data workflows are becoming essential for operational efficiency. This harmonized environment is accelerating digital adoption as sponsors seek systems that reduce administrative burden and ensure compliance. Consequently, vendors offering integrated, scalable, and EU-aligned eClinical solutions are gaining stronger traction in the region.
Restraint: Strict General Data Protection Regulation (GDPR) rules raise compliance and deployment costs
The stringent requirements of GDPR introduce substantial complexity for organizations implementing eClinical technologies in Europe. Sponsors and CROs must navigate strict rules for data processing, cross-border data transfers, consent management, and privacy safeguards. This increases legal and operational costs, particularly for multi-country studies with varying interpretations of the GDPR across local authorities. Vendors must invest in privacy design features, detailed documentation, and additional security controls, which lengthen deployment timelines. As a result, the GDPR remains a significant barrier that slows the pace of digital adoption and increases the total cost of ownership for eClinical systems.
Opportunity: Growth of RWD/RWE networks creates analytics opportunities
The expanding real-world data (RWD) and real-world evidence (RWE) networks in Europe, such as EHDEN and emerging HealthData@EU initiatives are creating new opportunities for advanced analytics within clinical research. Sponsors are increasingly looking to integrate clinical trial data with broader healthcare datasets to support decision-making, protocol optimization, and post-marketing evidence generation. This shift is driving demand for eClinical platforms that offer strong interoperability, data mapping, and analytics capabilities. Vendors that can enable seamless access to federated data sources and deliver actionable insights stand to benefit significantly, as these networks mature, the role of analytics-ready eClinical systems will become even more central to European research.
Challenge: Heavy validation requirements limit rapid vendor deployment
European authorities, ethics bodies, and research institutions anticipate extensive evaluation of eClinical systems, including detailed verification of software dependability, audit trails, and GxP compliance. This imposes a significant burden on manufacturers, particularly those adding new modules or deploying across numerous nations with different operational requirements. Extensive documentation, formal testing, and ongoing quality control can lead to implementation delays and increased project costs. Smaller vendors may struggle to meet these requirements efficiently, which reduces their competitiveness. Overall, high validation standards, while critical for data integrity and patient safety, remain a significant barrier to rapid growth in the European eClinical solutions industry.
EUROPE ECLINICAL SOLUTIONS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Providing CTR/CTIS-ready eClinical platforms, such as EDC, eConsent, and RTSM, that align with EU regulatory workflows and multi-country study requirements | Enables smoother EU-wide submission readiness| Accelerates study start-up across multiple Member States| Supports high compliance with European data and quality standards |
 |
Deploying its Unified Clinical Operations Suite, including CTMS, eTMF, and Study Startup, to support large and mid-size sponsors conducting trials across multiple European regions | Improves oversight of EU studies| Shortens site-activation timelines| Reduces operational inefficiencies across complex multi-country trials |
 |
Offering technology-enabled CRO services using eCOA, eSource, and analytics platforms adapted to GDPR requirements and multilingual European study environments | Ensures compliant data handling| Enhances the quality of patient-reported and site-entered data| Supports scalable execution of multi-country clinical programs |
|
|
Implementing hybrid and decentralized trial models with remote-monitoring technologies, digital recruitment, and site-support platforms tailored to European patient and site needs | Expands patient access across diverse EU populations| Reduces operational burden at sites| Increases recruitment and retention efficiency |
 |
Delivering validated eCOA, eConsent, and decentralized-trial solutions designed to meet European regulatory expectations and support multilingual deployment | Provides consistent patient and site experience across EU countries| Improves the accuracy of clinical outcome data| Streamlines consent and engagement processes |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe eClinical Solutions ecosystem is formed by a coordinated network of sponsors, CROs, technology providers, research locations, and regulatory agencies that collaborate within harmonized EU frameworks, as collaboration among pharmaceutical companies, digital health innovators, and data partners is hastening the transition to integrated clinical platforms. Academic medical centers and site networks are playing an increasingly important role as they utilize digital tools to expedite study execution and enhance data quality. This integrated ecosystem is increasingly focused on real-time data interchange, regulatory transparency, and scalable digital trial operations throughout the region.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe eClinical Solutions Market, By Product
As of 2024, the electronic data capture (EDC) & clinical data management solutions segment held the largest share of the Europe eClinical solutions market. Growth is being driven by the increasing need to standardize study data formats as sponsors prepare for the EMA’s evolving data submission requirements. The rise of complex and adaptive Phase III designs across Europe is prompting companies to utilize EDC systems that facilitate mid-trial adjustments without operational delays. The expansion of real-world data linkage initiatives in countries such as the Nordics and the UK is boosting demand for platforms that can integrate registry and EHR data securely. Cross-border trials under the EU CTR are creating pressure for centralized data oversight, strengthening the adoption of advanced clinical data management tools. Vendors are also seeing a rising demand for AI-enabled data cleaning and anomaly detection, which helps sponsors shorten database lock timelines.
Europe eClinical Solutions Market, By Deployment Model
The web-hosted & cloud-based models segment is projected to be the fastest-growing segment in the Europe eClinical solutions market during the forecast period. Growth is being fueled by the rising need for faster study start-up across multiple European regulatory jurisdictions, where cloud systems allow rapid configuration and rollout. Sponsors are also shifting to cloud platforms to support dynamic protocol amendments, which are becoming more common in oncology and rare disease trials. Increasing collaboration between European CROs and academic networks is driving demand for shared and multi-tenant environments that enable joint data access without complex infrastructure. The move toward EU-wide safety surveillance and real-time reporting is pushing companies to adopt cloud systems with stronger scalability and uptime guarantees. Additionally, cloud-based solutions are helping organizations manage distributed trial teams across Western and Eastern Europe, improving coordination and reducing IT overhead.
Europe eClinical Solutions Market, By Application
In Europe, the data collection segment held the largest share of the eClinical solutions market because the region is shifting toward digital, standardized, and audit-ready trial data. European sponsors and research sites rely on EDC, eCOA, ePRO, and eSource to streamline data capture and reduce transcription errors. These tools also improve transparency across multi-country studies. They play a major role in enhancing data quality and facilitating faster decision-making in complex therapeutic areas and geographically diverse trials. The growing use of mobile patient reporting tools, with automated data flows and integrated digital platforms that support multiple languages and regulatory needs, keeps data collection at the core of the European clinical research ecosystem.
Europe eClinical Solutions Market, By Clinical trial phase
By clinical trial phase, Phase IV is expected to grow at the fastest rate during the forecast period because Europe is expanding post-marketing evidence requirements. Regulatory bodies, such as the EMA, in collaboration with national competent authorities, now emphasize the importance of real-world evidence, long-term safety monitoring, and therapeutic performance assessment following market authorization. These expectations are increasing the number of Phase IV studies that measure treatment effectiveness in routine practice and track long-term risks. Sponsors must also fulfill pharmacovigilance duties by providing periodic safety updates and complying with post-authorization study obligations across EU Member States. The demand for eClinical platforms that support real-time safety tracking, continuous patient follow-up, and integration with European real-world data sources is increasing in the post-marketing landscape.
Europe eClinical Solutions Market, By end user
In Europe, pharmaceutical and biopharmaceutical companies accounted for the largest share of the eClinical Solutions market by end user. Growth is accelerating as many European pharma companies are expanding longitudinal cohort studies, which require robust platforms to manage multi-year data flows. The rise of country-specific reimbursement evidence requirements is pushing companies to invest in systems that can integrate clinical, economic, and quality of life data. Sponsors are also adopting eClinical tools to support portfolio-wide digital governance, ensuring consistent data standards across trials conducted in different EU regions. Additionally, Europe’s increasing focus on early access and conditional approval pathways is creating demand for faster data review cycles, which cloud-enabled eClinical platforms support efficiently.
REGION
Germany to be fastest-growing country in Europe eClinical Solutions market during forecast period
Germany is expected to register the highest CAGR during the forecast period. Growth is being driven by the rapid expansion of university hospital-led research networks, which increasingly require advanced digital platforms for coordinated data management. The country’s strong pipeline of precision medicine and immunotherapy trials is pushing sponsors to adopt systems that can manage complex biomarker and genomic datasets. The move toward digital hospital modernization (Krankenhauszukunftsgesetz – KHZG) is improving site-level readiness and accelerating the adoption of eClinical tools in the country. The rise of industry-insurer collaborations for real-world evidence generation is creating demand for platforms that can securely link trial data with health insurance datasets.
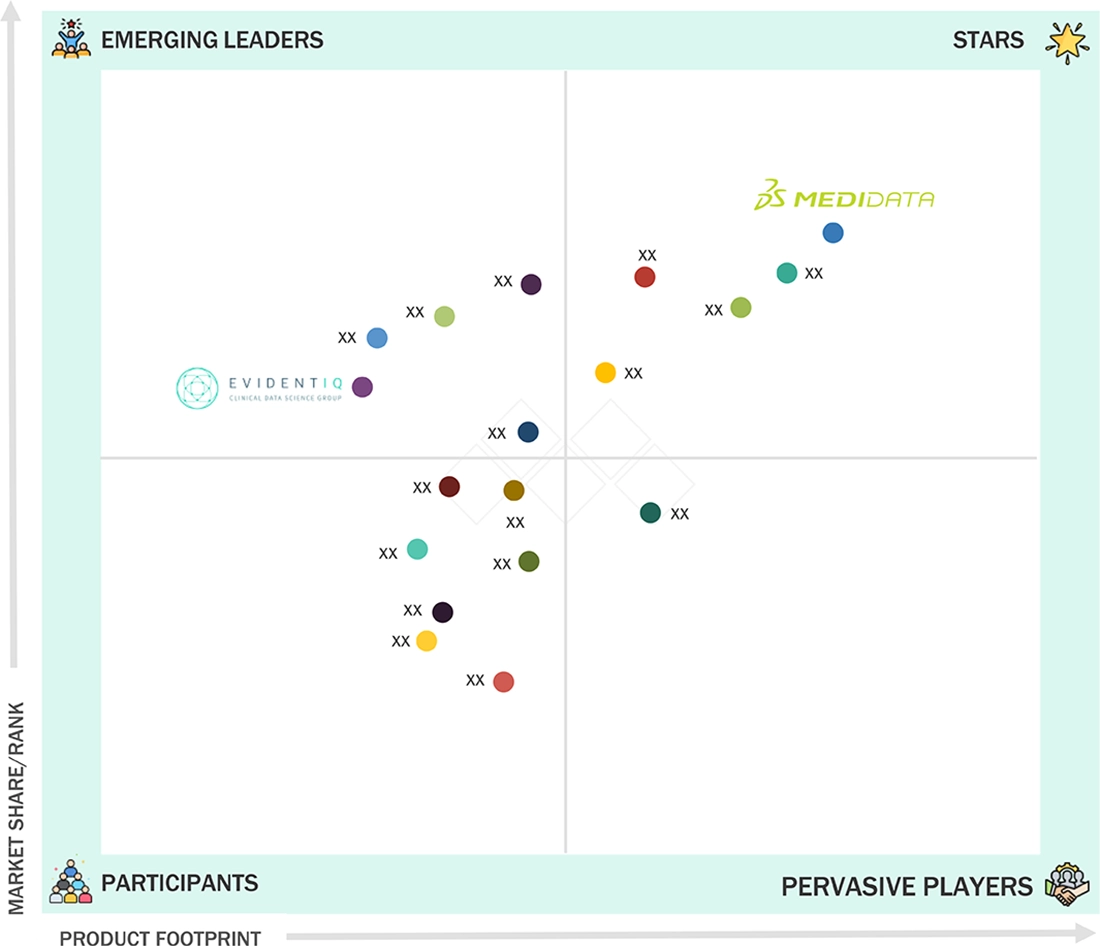
EUROPE ECLINICAL SOLUTIONS MARKET: COMPANY EVALUATION MATRIX
In the Europe eClinical solutions market matrix, Medidata (Star Player) leads with a dominant presence, as supported by its comprehensive eClinical platform and strong relationships with global and European pharma, as well as deep integration across EDC, eCOA, RTSM, and data analytics workflows. Its proven track record in managing complex, multi-country trials, combined with continuous investment in cloud-native and AI-enabled capabilities, reinforces its leadership across the region. EvidentIQ (Emerging Leader) is gaining strong traction with its European roots, expanding suite of eClinical tools, and growing adoption among mid-sized sponsors and academic research networks. While Medidata maintains a clear advantage through scale, maturity, and broad functional coverage, EvidentIQ demonstrates solid potential to move toward the leaders' quadrant as demand for locally aligned, GDPR-focused, and modular eClinical platforms increases across Europe.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Medidata (a Dassault Systèmes Company) (France)
- Veeva Systems (US)
- IQVIA (US)
- ICON Plc (Ireland)
- Oracle (US)
- Clario (US)
- ArisGlobal (US)
- Clinion (US)
- Parexel International (MA) Corporation (US)
- 4G Clinical (US)
- CompuGroup Medical (Germany)
- Saama (US)
- SOPHiA GENETICS (Switzerland)
- Advarra (US)
- Caidya (US)
- Qlucore (Sweden)
- EvidentlQ (Germany)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.82 Billion |
| Market Forecast in 2030 (Value) | USD 5.86 Billion |
| Growth Rate | CAGR of 13.0% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, France, UK, Italy, Spain, Rest of Europe |
| Parent & Related Segment Reports |
eClinical Solutions Market US eClinical Solutions Market Asia Pacific eClinical Solutions Market |
WHAT IS IN IT FOR YOU: EUROPE ECLINICAL SOLUTIONS MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Need for country-specific regulatory alignment across EU Member States | Provided segmentation and analysis tailored to CTR implementation, national ethics variations, and data-protection nuances | Improved strategic planning and faster market entry decisions across diverse EU regions |
| Requirement for GDPR-centric product and deployment insights | Developed GDPR impact framework, data-flow mapping, and vendor compliance benchmarking |
|
| Desire to understand competitive positioning of regional and global vendors | Delivered a Europe-specific competitive matrix, profiling capabilities, strengths, and adoption levels | Enabled clearer vendor selection, partnership targeting, and differentiated positioning |
| Request for detailed assessment of decentralized and hybrid trial adoption in Europe | Added country-level DCT readiness, infrastructure maturity insights, and technology adoption trends | Improved investment prioritization and product localization strategy |
RECENT DEVELOPMENTS
- January 2025 : Medidata (A Dassault Systèmes Company) (France) has launched the Clinical Data Studio, an AI-powered software platform designed to modernize clinical trial data management.
- January 2025 : Vita Global Sciences (US) partnered with Veeva Systems (US) to simplify clinical data management.
- October 2024 : Medidata (A Dassault Systèmes Company) (France) launched Rave Lite, which simplifies data collection and management, enabling research teams to conduct trials efficiently while ensuring compliance with regulatory standards.
- COLUMN 'A' SHOULD BE IN TEXT FORMAT AND NOT DATE FORMAT :
Table of Contents

Methodology
This research study involved the extensive use of both primary and secondary sources. It involved the analysis of various factors affecting the industry to identify the segmentation types, industry trends, key players, the competitive landscape of market players, and key market dynamics such as drivers, opportunities, challenges, restraints, and key player strategies.
Secondary Research
This research study extensively utilized secondary sources, including directories, databases such as Dun & Bradstreet, Bloomberg Businessweek, and Factiva, as well as white papers, annual reports, and companies' house documents. The aim of the secondary research was to gather and analyze information for a comprehensive and commercially focused study of the Europe eClinical Solutions Market, encompassing technical aspects and market dynamics. It also facilitated the identification of key players, market classification, industry trends, geographical markets, and significant market-related developments. Additionally, a database of prominent industry leaders was compiled through secondary research.
Primary Research
In the primary research process, various supply-side and demand-side sources were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side included industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, engineers, and related key executives from various companies and organizations operating in the Europe eClinical Solutions Market. Primary sources from the demand side included personnel from pharmaceutical & biotechnology companies, government organizations, research institutes and hospitals (small, medium-sized, and large hospitals).
Market Size Estimation
The total size of the Europe eClinical Solutions Market was determined after data triangulation through the two approaches mentioned below. After the completion of each approach, the weighted average of these approaches was taken based on the level of assumptions used in each approach.
Data Triangulation
The size of the Europe eClinical Solutions Market was estimated through segmental extrapolation using the bottom-up approach. The methodology used is as given below: -
- Revenues for individual companies were gathered from public sources and databases.
- Shares of leading players in the Europe eClinical Solutions Market were gathered from secondary sources to the extent available. In certain cases, shares of eClinical solutions businesses have been ascertained after a detailed analysis of various parameters including product portfolios, market positioning, selling price, and geographic reach & strength.
- Individual shares or revenue estimates were validated through interviews with experts.
- The total revenue in the Europe eClinical Solutions Market was determined by extrapolating the market share data of major companies.
Market Definition
eclinical solutions are the software/platform that changes the paper-based clinical research model into an electronic form. Such technologies help the researcher in facilitating the process of data collection, its transmission, and surveillance of the clinical trial process and provide enhanced options for better planning and execution of a clinical trial. eClinical technologies fast-track the study by reducing the risk and maximizing resources.
Key Stakeholders
- Healthcare IT Service Providers
- eClinical Solution Vendors
- Clinical Research Organizations
- Pharmaceutical/Biopharmaceutical Companies
- Research and Development (R&D) Companies
- Business Research and Consulting Service Providers
- Medical Research Laboratories
- Government agencies
- Healthcare startups, consultants, and regulators
- Academic Medical Centers/Universities/Hospitals
Objectives of the Study
- To define, describe, and forecast the Europe eClinical Solutions Market based on product, deployment model, application, clinical trial phase, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To analyze the micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall Europe eClinical Solutions Market
- To analyze the opportunities for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players and analyze their market shares and core competencies2
- To track and analyze competitive developments such as product launches & approvals, partnerships, agreements, and collaborations in the overall Europe eClinical Solutions Market
- To benchmark players within the market using the proprietary "Competitive Leadership Mapping" framework, which analyzes market players on various parameters within the broad categories of business and product strategy.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to 5)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe eClinical Solutions Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Europe eClinical Solutions Market