
Asia Pacific Pharmaceutical Drug Delivery Market Size, Growth, Share & Trends Analysis
Asia Pacific Pharmaceutical Drug Delivery Market by Route of Administration (Oral, Injectors (Auto Injectors), Implantable, Transmucosal, Nasal, Syringes), Application (Cancer, Diabetes), Care Setting (Hospital, Home Care) - Forecast to 2031



OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
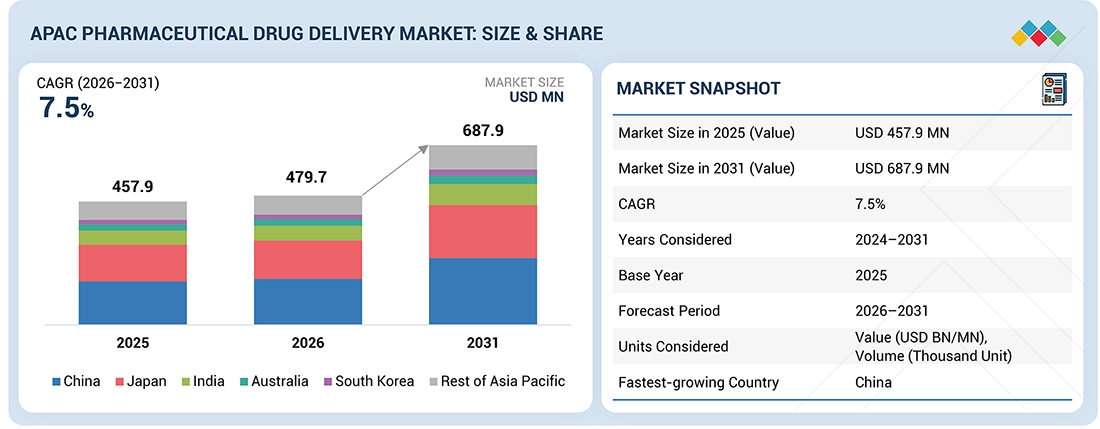
The Asia Pacific pharmaceutical drug delivery market, valued at US$457.9 million in 2025, stood at US$479.7 million in 2026 and is projected to advance at a resilient CAGR of 7.5% from 2026 to 2031, culminating in a forecasted valuation of US$687.9 million by the end of the period. The market growth for drug delivery in the APAC region is steadily expanding due to an increase in disease prevalence, an increase in the adoption rate of biologics, advancements in technologies, and an increase in regional production.
KEY TAKEAWAYS
-
By CountryChina is projected to witness the highest growth rate of 7.8% during the forecast period in the APAC pharmaceutical drug delivery market.
-
By Route of AdministrationBy route of administration, the injectable drug delivery segment holds the largest share in 2025 in this market.
-
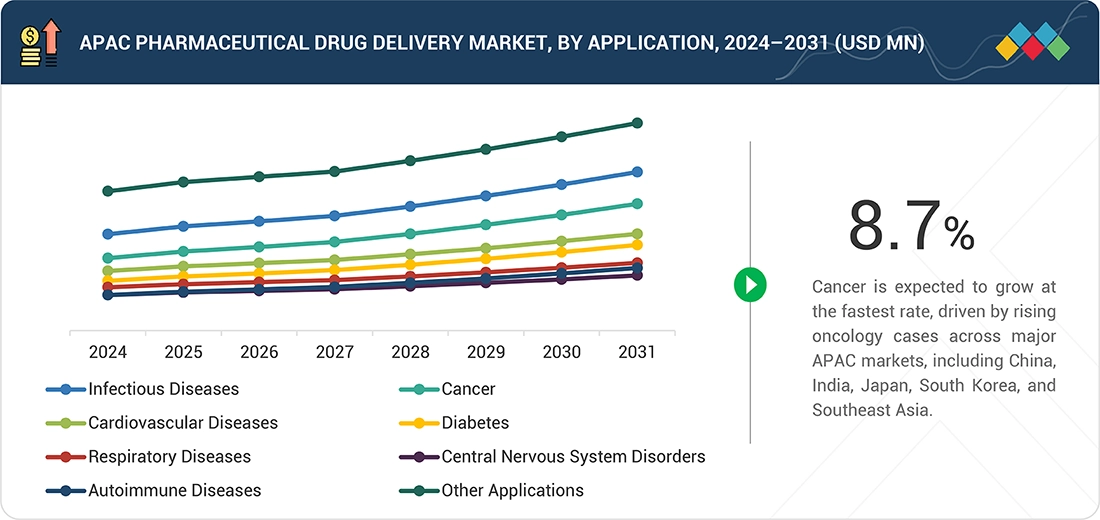
By ApplicationBy application, cancer is projected to experience the highest growth rate from 2026 to 2031.
-
By Facility of UseBy facility of use, the hospital segment holds the largest share in 2025 in the APAC pharmaceutical drug delivery market.
-
Competitive Landscape - Major PlayersTerumo and Pfizer lead the APAC market through advanced delivery innovations, strong regional presence, and sustained investment.
-
Competitive Landscape - StartupsStartups such as Daewoong Therapeutics (South Korea), Nusantics (Indonesia), and ACM Biolabs (Singapore) are gaining prominence in the APAC pharmaceutical drug delivery market due to their innovative platforms, and focus on next-generation delivery technologies.
Growth factors will also aid market expansion in the APAC pharmaceutical drug delivery market, driven by the growing elderly population in the region, increasing awareness of treatment adherence, and the rise in demand for home-based and self-administration solutions. Urbanization and increased diagnosis rates are driving therapeutic volumes, and with digital health ecosystems receiving immense investments, the adoption of connected delivery devices is gaining momentum. Partnerships between global pharma companies and APAC CDMOs are further strengthening the market growth.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The APAC market for pharmaceutical drug delivery solutions is undergoing rapid transformation, driven by an increase in the use of biologics and biosimilars. The adoption rate of IoT devices, microneedle patches, nanoparticles, and smart inhalers is significantly impacting patient care. Simultaneously, there is an ascending demand for self-administration, scaling up production, and compliance with government standards, prompting vendors to develop more automated and compliant drug delivery solutions.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising prevalence of chronic diseases

-
Increasing adoption of connected and self-administration devices
Level
-
Wide disparities in regulatory maturity and approval timelines
-
High-cost sensitivity in emerging markets
Level
-
Growing demand for wearable injectors, and home-care delivery solutions
-
Government investments in digital health
Level
-
High competition from low-cost local players
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising prevalence of chronic diseases
The rising number of incidences related to diabetes, cardiovascular disease, respiratory disease, and cancer is, in turn, fueling a considerable market demand for modern drug delivery systems in the APAC region. Rapid urbanization and a shift in lifestyles and demographics, driven by an aging population and improved diagnostic facilities, are leading to an acceleration in disease prevalence, most notably in China, India, and Southeast Asia. Nevertheless, an increasing burden of chronic illness and governmental initiatives are fueling market demand for injectable drug delivery systems and inhalation therapies.
Restraint: Wide disparities in regulatory maturity and approval timelines
Countries within the APAC region exhibit marked divergences in terms of regulatory competence, resulting in inconsistent approval timelines for drug-delivery devices and combination products. Developed regions, such as Japan, Singapore, and Australia, have efficient and transparent regulatory systems, whereas developing regions, including Indonesia, Vietnam, and the Philippines, have limited regulatory capabilities and changes in regulatory standards that result in an extended timescale. These factors hamper product releases within the region and make it challenging for businesses with operations across several regions.
Opportunity: Growing demand for wearable injectors, and home-care delivery solutions
The adoption of wearable injectors and home care delivery systems is increasing at a rapid rate in the APAC region as there is a transition taking place in healthcare, and it is becoming more decentralised. The burden on the hospitals and prevalence of chronic diseases and biologics usage are additionally accelerating adoption. Also, urbanisation and the adoption of digital health and better reimbursements in mature economies of the APAC region are encouraging adoption. Home-based care is also a crucial solution for efficient patient management.
Challenge: High competition from low-cost local players
A major challenge in the APAC market for pharmaceutical drug-delivery systems is tough competition from low-cost regional participants. Local competitors, who have established operations in China, India, and Southeast Asia, have been offering syringes, infusion sets, and basic drug-delivery systems at highly competitive prices. Due to economies of scale and regional low-cost operations, regional competitors have been able to offer low-cost pricing, making it very challenging for global competitors to match these costs.
ASIA PACIFIC PHARMACEUTICAL DRUG DELIVERY MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Utilizes advanced platforms like OROS, patches, pumps, and controlled-release systems to support integrated drug–device solutions across major therapeutic areas | Diverse delivery technologies improve adherence, enable long-acting release, and support flexible drug–device combinations for biologics and complex therapies |
 |
Develops oral, injectable, sustained-release, and lipid nanoparticle systems, including mRNA delivery technologies, supported by strong formulation and controlled-release expertise | Broad delivery formats enhance treatment consistency, support advanced biologics, and enable rapid adoption of innovative nanoparticle-based drug delivery technologies |
 |
Provides precision injectables, infusion systems, pre-fillable syringes, and catheter-based platforms, supporting safe and reliable parenteral drug delivery across APAC markets | High-quality systems improve dosing accuracy, support biologics and vaccines, and strengthen pharma partnerships through reliable drug–device integration capabilities |
 |
Expands inhalation, injectable biologics, and subcutaneous delivery platforms while advancing microencapsulation and manufacturing for radioligand and next-generation therapies | Patient-friendly delivery routes enhance adherence, while expertise in complex biologics and radioligand therapies strengthens leadership in precision medicine |
 |
Offers diverse oral, injectable, antiviral, oncology, and nano-enabled delivery systems supporting complex molecules and high-demand biologics therapies | Versatile delivery solutions improve adaptability across therapies, supporting biologics growth and ensuring reliable, high-quality drug delivery performance |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
Collaboration among pharmaceutical companies, medical device manufacturers, CDMOs, and digital health platforms forms the APAC pharmaceutical drug delivery ecosystem, which can adopt advanced delivery technologies quickly. Hospitals, regulators, and emerging startups will further strengthen this ecosystem by fostering innovation, enhancing patient access, and localized manufacturing for injectables, biologics, and next-generation delivery systems.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
By Application
By application, the cancer segment is expected to show the highest growth due to an enormous acceleration in cancer instances within China, India, Japan, South Korea, and Southeast Asia. There will be an accelerated adoption rate of cancer therapies involving targeted therapies, immunotherapy, and biologics, all of which demand sophisticated drug delivery systems. Governments and healthcare infrastructure are strengthening cancer care, enhancing screening facilities, and improving access to innovative therapies. At the same time, advancements and investments within injectable drug formulations, sustained releases, and combination drug devices made within the region’s pharma sector and CDMOs will fuel an accelerated growth rate within this sector.
By Facility of Use
By facility of use, the hospital sector presently retains the predominant market share in the pharmaceutical market in 2025 within the APAC region, due to the region's large dependence on hospital-based delivery of injectable, infusion, and cancer therapies. Hospitals across China, Japan, and India continue to expand and improve their facilities and capabilities with modern drug delivery systems and manage large volumes of patients. Additionally, government spending on developing cancer and critical care facilities maintains the hospital sector in a leading position. Moreover, hospitals remain at the core of delivering and managing risk-associated therapies.
By Route of Administration
By route of administration, injectable pharmaceutical drug delivery is expected to hold the largest market share in the Asia Pacific pharmaceutical drug delivery market in 2025, driven by the increasing demand for biologics, biosimilars, vaccines, and therapies for chronic diseases, which typically require parenteral routes. Moreover, there has been an added need for subcutaneous and IV-based drug delivery solutions because of rising incidences of Chronic Diseases like Diabetes, Cancer, and Autoimmune Diseases. Additionally, due to the high adoption rates of pre-filled syringes, auto-injectors, infusion devices, and wearable injectors, it is expected to maintain a dominant position. Moreover, advances in hospital infrastructure, government initiatives for immunizations, and the swift growth of CDMOs for Injectable drug developments and productions from APAC have added value to it.
REGION
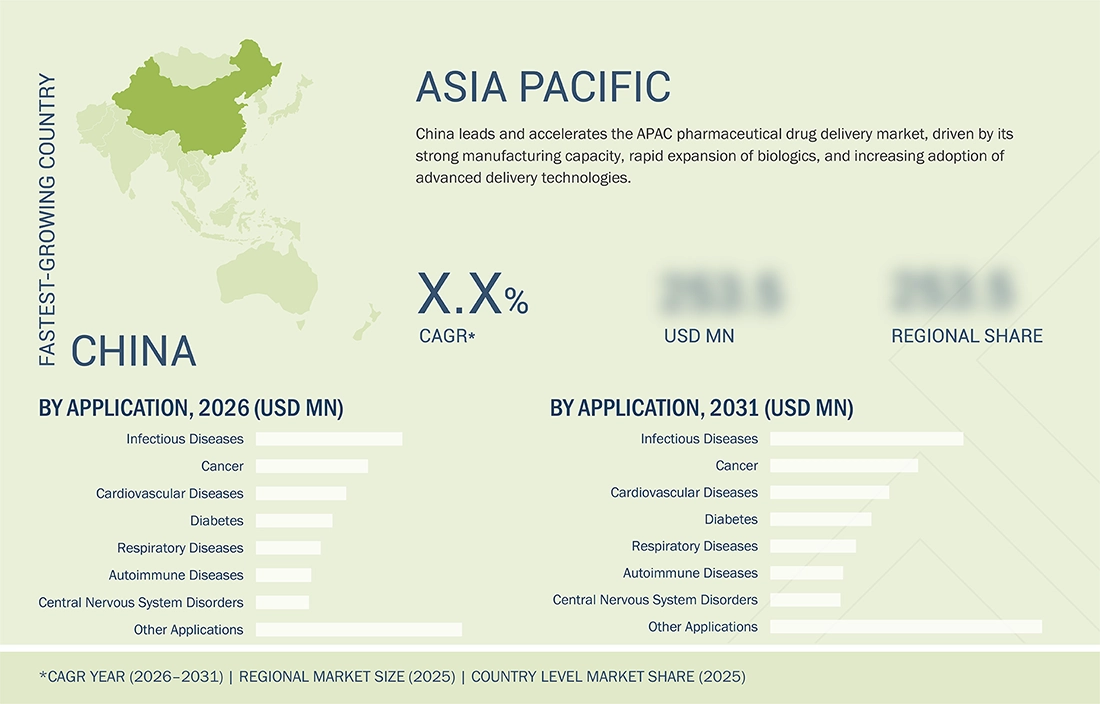
By Region
China leads the APAC pharmaceutical drug delivery market in 2025, driven by strong pharma manufacturing, growing demand for biologics and biosimilars, and expanding vaccination programs. It is also expected to grow the fastest from 2026 to 2031, driven by rising healthcare investment, the adoption of advanced delivery technologies, and an increasing chronic disease burden, which will further boost the use of injectables, inhalation, and transdermal systems.
ASIA PACIFIC PHARMACEUTICAL DRUG DELIVERY MARKET: COMPANY EVALUATION MATRIX
Terumo stands out as a Star Player within the APAC pharmaceutical drug delivery market based on its large regional production base and expertise, and historic relationships with drug manufacturers in Asia. Its precision-engineered drug-delivery solutions make it a sought-after alternative within rapidly expanding biologics and vaccine markets. On the flip side, Pfizer transitions from a market challenger to an emerging leader based on its growing presence within the broader APAC market as a result of its advances within special formulations, mRNA, and nanoparticle drug-delivery technologies.
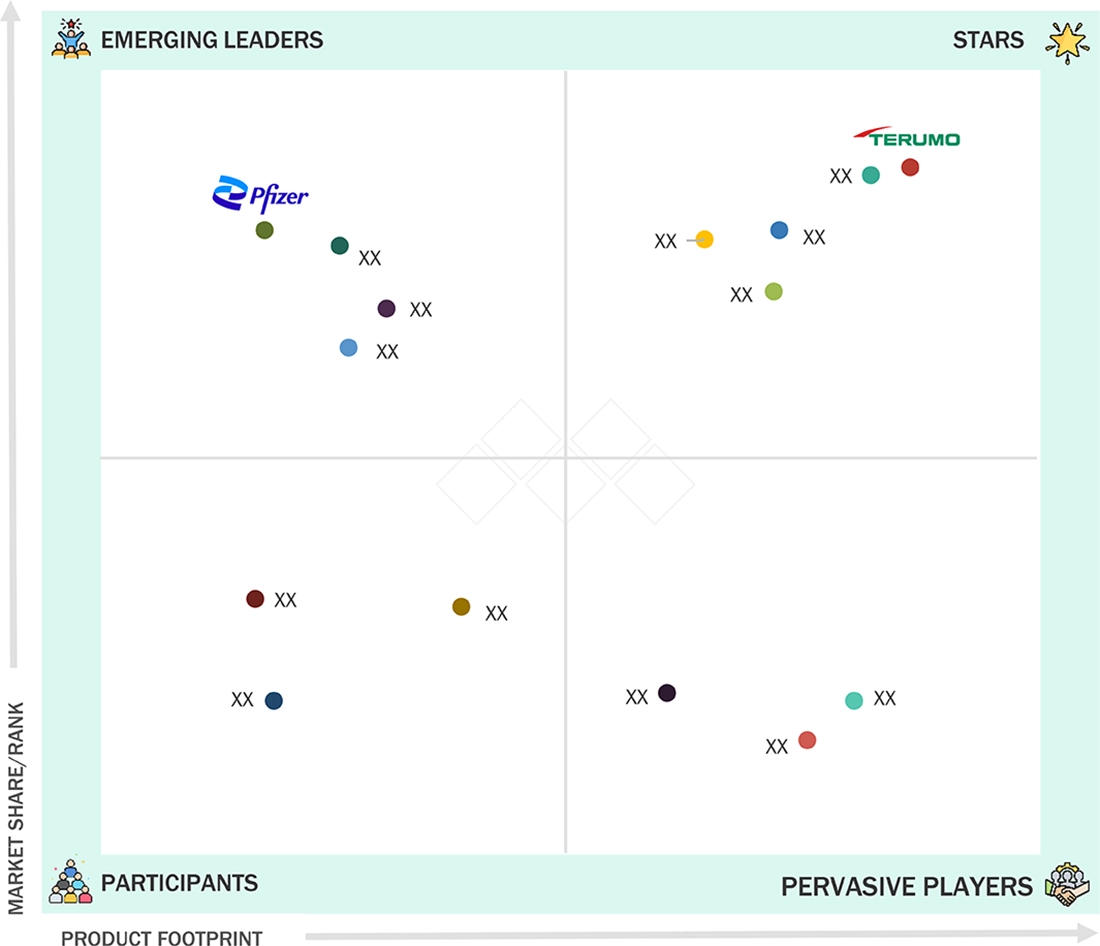
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Johnson & Johnson Services, Inc. (US)
- Pfizer Inc. (US)
- Terumo Corporation (Japan)
- Becton, Dickinson and Company (US)
- Merck & Co., Inc. (US)
- B. Braun (Germany)
- Gerresheimer AG (Germany)
- Cipla (India)
- Eli Lilly and Company (US)
- Novartis (Switzerland)
- Biogen (US)
- Baxter International (US)
- West Pharmaceutical Services (US)
- Sun Pharmaceutical Industries (India)
- GlaxoSmithKline (GSK) (UK)
- Semex (Canada)
- Superior Animal Genetics (India)
- XytoVet (Australia)
- Swine Genetics International (US)
- Generatio GmbH (Germany)
- Cogent Breeding (UK)
- Weatherbys Scientific (Ireland)
- GenSol Diagnostics, LLC (US)
- Sandor Animal Biogenics Pvt. Ltd. (India)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 457.9 MN |
| Market Size in 2031 (Value) | USD 687.9 MN |
| CAGR | 7.5% during 2026-2031 |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD MN), Volume (Thousands Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regional Scope | China, Japan, India, Australia, South Korea and Rest of APAC |
| Parent & Related Segment Reports |
Pharmaceutical Drug Delivery Market Europe Pharmaceutical Drug Delivery Market US Pharmaceutical Drug Delivery Market |
WHAT IS IN IT FOR YOU: ASIA PACIFIC PHARMACEUTICAL DRUG DELIVERY MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Assessed key drug-delivery types in APAC—oral, injectable, inhalation, transdermal, implantable, nanoparticle-based, and connected devices. Evaluated performance, regulatory expectations, and innovation trends used by leading manufacturers | Helped clients select suitable delivery technologies, compare long-acting and device–drug options, and understand emerging platforms such as mRNA systems and smart injectors. Supported product planning for chronic disease and biologics-focused treatments |
| Company Information | Profiled major global and APAC drug-delivery players, covering portfolios, platforms, partnerships, manufacturing, and R&D efforts. Analyzed strengths in biologic and sustained-release delivery | Enabled clients to gauge competitor capabilities, assess supplier fit for injectables and device partners, and identify collaboration or outsourcing opportunities. Strengthened long-term strategy in biologics and advanced delivery solutions |
| Geographic Analysis | Examined APAC delivery trends across regulatory systems, biologics expansion, home-care adoption, and manufacturing hubs. Included reimbursement and digital-health ecosystem insights, with optional country-level customization | Supported strategic market entry by identifying growth areas, localization options, and supply-chain opportunities. Helped clients evaluate patient preferences and emerging demand in home-based and biologics-driven delivery segments across diverse APAC markets |
RECENT DEVELOPMENTS
- February 2024 : AstraZeneca acquired Gracell Biotechnologies to expand its pipeline of advanced cell therapies, strengthening its innovation capabilities and presence in China
- October 2023 : Boehringer Ingelheim partnered with Precision Health Research to drive precision healthcare advancements in Singapore and improve patient outcomes across APAC
- July 2023 : Bayer collaborated with Peking University to accelerate drug discovery and translational research, focusing on oncology, cardiometabolic diseases, immunology, and cell & gene therapies in China
- August 2022 : Sanofi and Innovent Biologics entered an agreement to hasten the development of oncology medicines and strengthen Sanofi’s commercial footprint in China
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, fundamental market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), European Federation of Pharmaceutical Industries and Associations (EFPIA), American Journal of Drug Delivery and Therapeutics, Center for Drug Delivery and Nanomedicine (CDDN) and Parenteral Drug Association (PDA) were referred to identify and collect information for the asia pacific pharmaceutical drug delivery market study. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the asia pacific pharmaceutical drug delivery market. The primary sources from the demand side include hospitals, ambulatory surgical centers/clinics, home care setting, diagnostic centers among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
Market Estimation Methodology
For the APAC market value, annual revenues were calculated based on the revenue mapping of major product manufacturers and OEMs active in the asia pacific pharmaceutical drug delivery market. All the major product manufacturers were identified at the APAC and/or country/regional level. Revenue mapping for the respective business segments/sub-segments was done for the major players (who contribute at least 45-48% of the market share at the APAC level). Also, the asia pacific pharmaceutical drug delivery market was split into various segments and sub-segments based on:
- List of major players operating in the asia pacific pharmaceutical drug delivery products market at the regional and/or country level
- Product mapping of various asia pacific pharmaceutical drug delivery manufacturers at the regional and/or country level
- Mapping of annual revenue generated by listed major players from asia pacific pharmaceutical drug delivery (or the nearest reported business unit/product category)
- Revenue mapping of major players to cover at least 45% of the APAC market share as of 2023
- Extrapolation of the revenue mapping of the listed major players to derive the APAC market value of the respective segments/subsegments
- Summation of the market value of all segments/subsegments to arrive at the APAC point-of-care diagnostics market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
Data Triangulation
After arriving at the overall size of the Asia Pacific pharmaceutical drug delivery market through the above-mentioned methodology, this market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value data for the key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Market Definition
Drug delivery is a method or process of administration of a pharmaceutical drug to safely attain its desired therapeutic effect. Advancements in drug delivery offer several benefits, such as ease of use, convenience, and patient compliance. Drug delivery technologies are used for the targeted delivery and/or controlled release of therapeutic agents.
Key Market Stakeholders
- Pharmaceutical manufacturing companies
- Original equipment manufacturing companies
- Suppliers and distributors of pharmaceutical products and medical devices
- Healthcare service providers
- Teaching hospitals and academic medical centers
- Health insurance players
- Government bodies/municipal corporations
- Regulatory bodies
- Medical research institutes
- Business research and consulting service providers
- Venture capitalists
- Market research and consulting firms
Report Objectives
- To define, describe, and forecast the Asia Pacific pharmaceutical drug delivery market on the basis of asia pacific pharmaceutical drug delivery product, route of administration, application, facility of use, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for key stakeholders and provide details of the competitive landscape for major market leaders
- To profile the key market players and comprehensively analyze their market shares and core competencies2
- To track and analyze competitive developments such as mergers and acquisitions, new product developments, partnerships, agreements, collaborations, and expansions in the asia pacific pharmaceutical drug delivery market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the asia pacific pharmaceutical drug delivery market report.
Product Analysis
- Product Matrix, which gives a detailed comparison of the product portfolios of the top five APAC players.
Company Information
- Detailed analysis and profiling of additional market players (up to 5 players)
Geographic Analysis
- Further breakdown of the Rest of Europe pharmaceutical drug delivery market into Belgium, Austria, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal, among other
- Further breakdown of the Rest of Asia Pacific pharmaceutical drug delivery market into New Zealand, Vietnam, Philippines, Singapore, Malaysia, Thailand, and Indonesia, among other countries
- Further breakdown of the Rest of Latin America pharmaceutical drug delivery market into Argentina, and Colombia, among other countries.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific Pharmaceutical Drug Delivery Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation














Growth opportunities and latent adjacency in Asia Pacific Pharmaceutical Drug Delivery Market