
Europe Pharmaceutical Drug Delivery Market Size, Growth, Share & Trends Analysis
Europe Pharmaceutical Drug Delivery Market by Route of Administration [Oral (Tablet, Capsule), Injector, Topical (Cream, Transdermal Patch), Transmucosal, Nasal], Application [Cancer, Diabetes], Care Setting [Hospital, Homecare]- Forecast to 2031




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
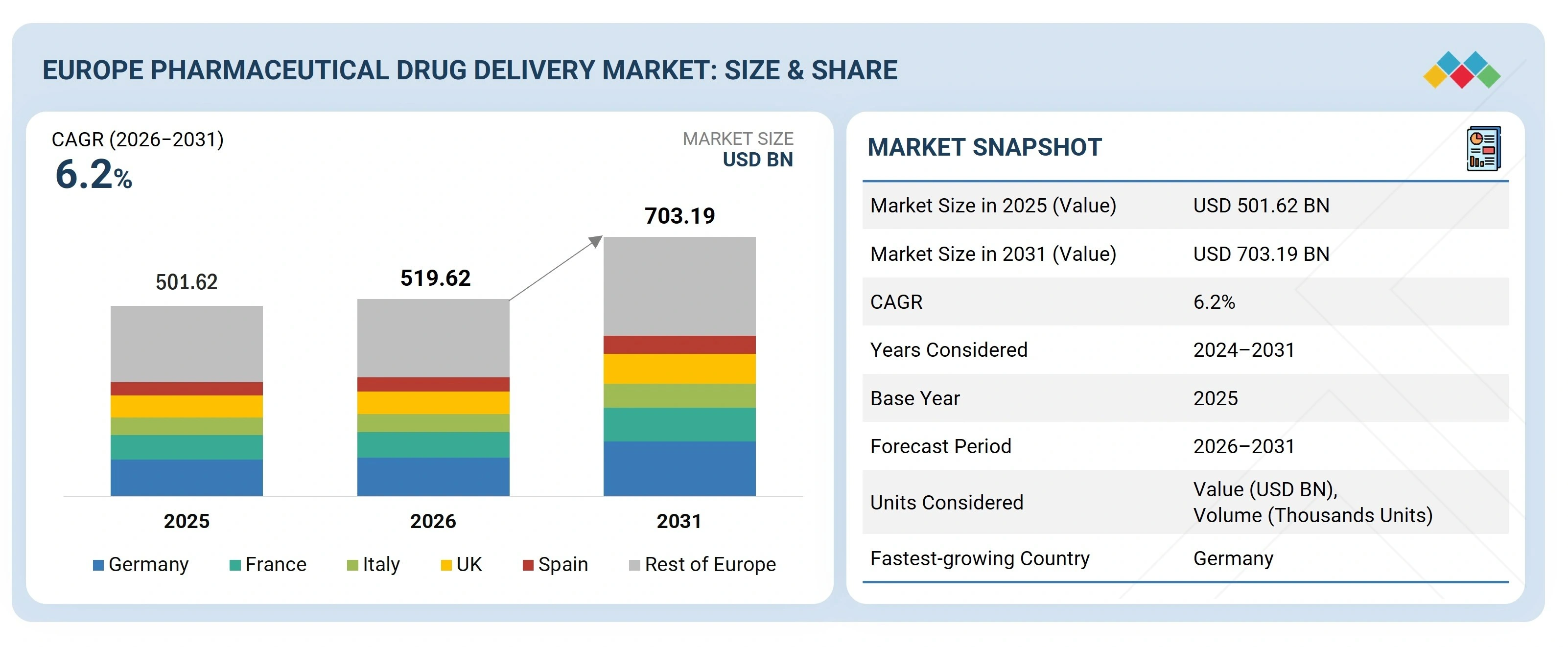
The Europe Pharmaceutical drug delivery market, valued at USD501.62 billion in 2025, stood at USD519.62 billion in 2026 and is projected to advance at a resilient CAGR of 6.2% from 2026 to 2031, culminating in a forecasted valuation of USD703.19 billion by the end of the period. The market growth is driven by rising use of biologics and biosimilars, an increasing prevalence of chronic diseases, and growing acceptance of innovative drug delivery devices, including auto-injectors, prefilled syringes, and inhalation systems. An aging population, rapid adoption of home care, and supportive regulatory policies promoting patient-friendly drug delivery systems are also fueling this growth.
KEY TAKEAWAYS
-
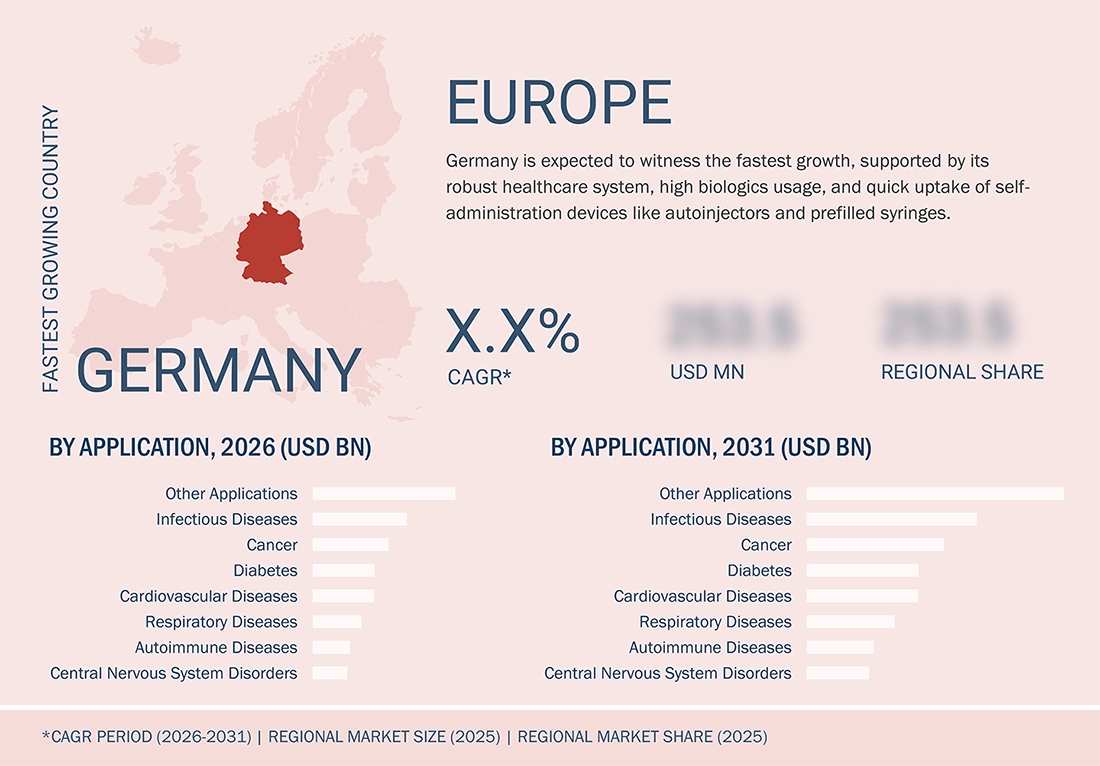
By CountryGermany is expected to grow at a CAGR of 7.2% during the forecast period.
-
By Route of AdministrationThe injectable drug delivery segment held the largest market share of 38.0% in 2025.
-
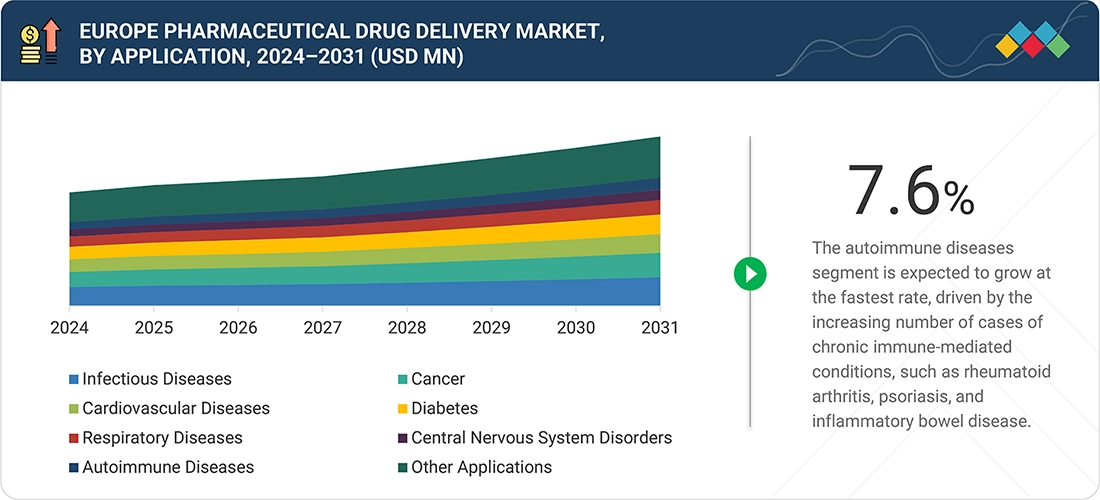
By ApplicationThe autoimmune diseases segment is expected to record the highest CAGR of 7.6% from 2026 to 2031.
-
By care settingThe hospitals segment held the largest market share of 58.8% in 2025.
-
Competitive Landscape - Major PlayersGerresheimer AG (Germany) and Novo Nordisk (Denmark) stand out as star players in Europe because of their strong regional footprint, established product portfolios, and consistent leadership in advancing drug delivery technologies.
-
Competitive Landscape - StartupsCapa Valve Ltd (Belgium) and Pharma Latch (UK) are emerging players, gaining attention for their novel approaches to improving precision, ease of use, and patient experience in drug delivery.
In Europe, there is a rising interest in innovative drug delivery systems as healthcare systems seek ways to improve drug administration. Germany, France, and the Nordic countries are leading this effort with their healthcare budgets. Meanwhile, new development policies and high sustainability demands are driving innovations in device development to create patient-focused and sustainable drug delivery solutions.
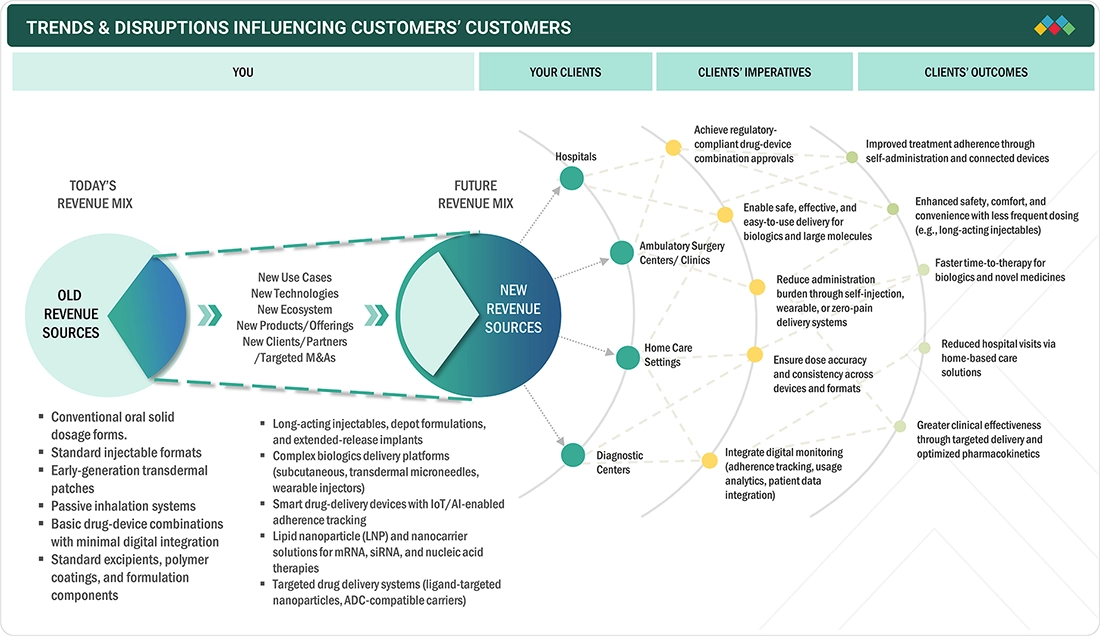
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
Some emerging trends in Europe include the increased popularity of sustainable drug delivery packaging, the use of self-administration devices, and improved integration of digital functionalities for compliance management. These trends are impacting the market as companies are forced to innovate rapidly due to stricter government policies in the European Union.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Prevalence of chronic diseases

-
Increased adoption of connected and self-administration devices
Level
-
Wide disparities in regulatory maturity and approval timelines
-
High-cost sensitivity in emerging markets
Level
-
Surge in demand for wearable injectors and home-care delivery solutions
-
Government investments in digital health
Level
-
Intense competition from low-cost local players
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Prevalence of chronic diseases
The increasing prevalence of chronic diseases such as diabetes, cardiovascular diseases, respiratory illnesses, and cancer in Europe has driven greater demand for advanced drug delivery devices. The rise in new cases of chronic conditions in Western and Northern Europe, along with their growing use of biologics in medical practice, has further boosted the need for injectable, respiratory, long-acting, and self-administered devices.
Restraint: Wide disparities in regulatory maturity and approval timelines
The relatively organized but distinct regulatory system in Europe leads to different timelines for approving drug delivery devices and combination products across various EU member countries. While Germany, France, and Nordic countries have streamlined review processes, other nations in Southern and Eastern Europe often take longer for device approval, and compliance with device standards in those areas is still developing.
Opportunity: Surge in demand for wearable injectors and home-care delivery solutions
The trend of wearable injectors is driven by a shift toward decentralized care, integration with digital health, and self-administration solutions as health systems aim to reduce hospital burdens. The increasing use of biologics, supported by reimbursement pathways in major EU markets, along with rising investments in connected drug delivery platforms, further boosts this growth. Additionally, the expansion of home care adoption among aging populations, combined with the EU's efforts on harmonized device regulations, creates new opportunities for market growth across Europe's diverse healthcare systems.
Challenge: Intense competition from low-cost local players
Intense competition from low-cost regional sources challenges the Europe pharmaceutical drug delivery market, especially in commoditized product lines such as syringes, infusion sets, and other delivery kit components. The market faces potential import threats from Turkey and low-cost options from manufacturers in Central and Eastern European countries. The main drawback of this low-cost competition model is that it hampers innovation in niche technologies, resulting in limited returns on investment for established market leaders.
EUROPE PHARMACEUTICAL DRUG DELIVERY MARKET SIZE, GROWTH, SHARE & TRENDS ANALYSIS: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Builds a strong portfolio of injection-based delivery solutions, including smart pens, autoinjectors, and connected devices designed for diabetes, obesity management, and chronic care needs across Europe. | These intuitive delivery systems simplify self-treatment, support accurate dosing, and help improve day-to-day therapy adherence, aligning well with Europe’s growing shift toward home-based care. |
 |
Offers a diverse set of delivery systems across oral, injectable, inhalation, and long-acting preparations, with particular emphasis on biologics, vaccines, and chronic therapeutic areas. | This breadth provides flexibility across disease areas, supports rapid deployment of new therapies, and ensures reliable drug performance for Europe’s varied healthcare environments. |
 |
Specializes in precision-engineered primary packaging and drug delivery components, including prefillable syringes, inhalation parts, and device-integrated solutions tailored for European regulatory and manufacturing needs. | High-performance components elevate dosing reliability, enhance safety standards, and enable seamless integration into pharma partners’ combination products, reinforcing Gerresheimer’s role as a key enabler for EU biologics and injectables. |
 |
Strengthens its delivery landscape through advanced inhalation technologies, subcutaneous biologic platforms, and targeted delivery systems for next-generation therapies. | These innovations support more convenient treatment pathways, improve patient comfort, and demonstrate strong leadership in designing delivery systems suited for complex, evolving therapeutics. |
 |
Advances a wide mix of delivery formats—from oral and injectable options to lipid nanoparticle and mRNA-based systems—supported by deep formulation and controlled-release capabilities. | This broad delivery toolbox allows Pfizer to support innovative biologics and vaccines while ensuring consistent, efficient drug administration for large patient populations across Europe. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The strong network of device manufacturers, global and regional pharmaceutical companies, and advanced healthcare institutions drives the adoption of innovative delivery systems, shaping Europe's pharmaceutical drug delivery landscape. This is supported by a well-established regulatory framework led by EMA, MHRA, and national authorities to ensure that safety standards, evaluation of combination products, and market access are high.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
By Application
The rapid growth in the autoimmune diseases segment is fueled by increasing cases of rheumatoid arthritis, psoriasis, and inflammatory bowel disease. As more biologic and targeted therapies enter the market, many with injectable or infusion methods, demand for advanced delivery systems continues to rise. Improved diagnosis, greater treatment access, and a trend toward self-injectable therapies all boost the need for specialized drug delivery solutions in autoimmune disease management.
By Care Setting
The dominance of hospitals can be attributed to their high volume of acute care, infusion, and complex biologics that require monitoring. Hospitals in Europe benefit from a stronger purchasing position in their markets and a preference for adopting advanced infusion pumps, injectables, and controlled delivery systems early. Increasing surgical volumes, cancer cases, and emergency care also reinforce that drug delivery remains in a hospital setting, despite the development of home healthcare options.
By Route of Administration
Injectables represent the largest segment, driven by increased European reliance on biologics and biosimilars for cancer, autoimmune, and chronic conditions. These prefilled injectables in syringe, auto-injector, or pen form remain popular due to their ease of use, accuracy, and portability. The growing acceptance of patient self-administration, along with support for developing combination products, further confirms injectables as the most favored administration method in Europe.
REGION
By Region
Germany is expected to experience the fastest growth rate, with a well-functioning healthcare infrastructure, a high proportion of biologic prescriptions, and a rapid adoption rate of user-controlled devices such as auto-injectors and prefilled syringes. The country has a sizable number of seniors, an extensive hospital network, and favourable government policies that encourage the usage of superior delivery systems. Additionally, its long-standing strength in R&D and manufacturing in the pharmaceutical domain keeps promoting injectable, inhalation, and wearable delivery systems for various therapy classes.
EUROPE PHARMACEUTICAL DRUG DELIVERY MARKET SIZE, GROWTH, SHARE & TRENDS ANALYSIS: COMPANY EVALUATION MATRIX
Novo Nordisk is recognized as a star player in the EU pharmaceutical drug delivery market due to its strong leadership in injectable and pen-based delivery systems, extensive biologics portfolio, and broad commercial presence across major European countries. Its pace of innovation and consistent market expansion reinforce its top-tier position. Meanwhile, Sanofi is seen as an emerging leader, supported by its growing inhalation and injectable delivery platforms, increased investment in combination products, and rising competitiveness in diabetes, oncology, and immunology therapies across Europe.
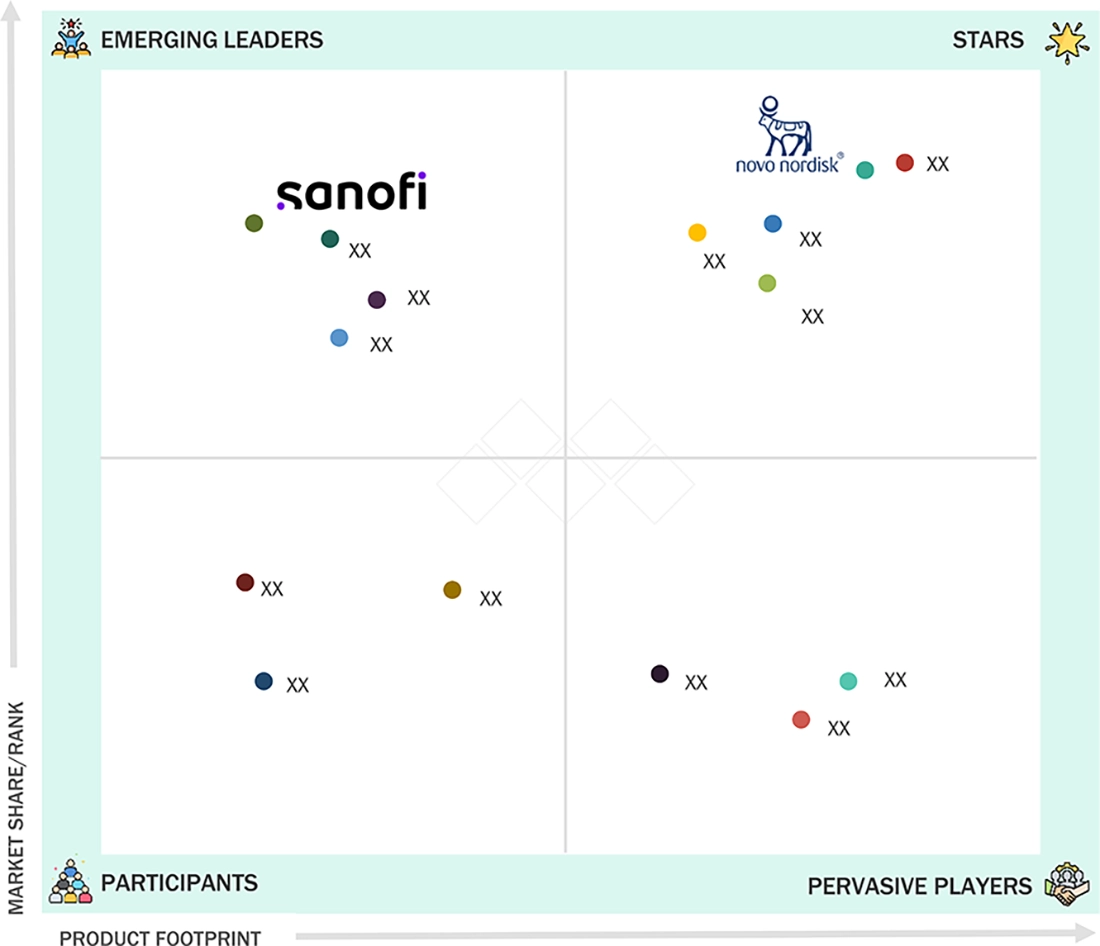
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Johnson & Johnson Services, Inc. (US)
- Pfizer Inc. (US)
- Fresenius Kabi (Germany)
- Becton, Dickinson and Company (US)
- Nemera (France)
- B. Braun (Germany)
- Gerresheimer AG (Germany)
- SHL Medical (Switzerland)
- Novo Nordisk (Denmark)
- Novartis (Switzerland)
- Sanofi (France)
- Baxter International (US)
- AstraZeneca (UK)
- Roche (Switzerland)
- medmix (Switzerland)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 501.62 BN |
| Market Size in 2031 (Value) | USD 703.19 BN |
| CAGR | 6.2% |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD BN), Volume (Thousands Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regional Scope | Germany, France, Italy, UK, Spain, Netherlands, Belgium, Sweden, Rest of Europe |
| Parent & Related Segment Reports |
Pharmaceutical Drug Delivery Market US Pharmaceutical Drug Delivery Market APAC Pharmaceutical Drug Delivery Market |
WHAT IS IN IT FOR YOU: EUROPE PHARMACEUTICAL DRUG DELIVERY MARKET SIZE, GROWTH, SHARE & TRENDS ANALYSIS REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis | Assessed key drug-delivery types in Europe—oral, injectable, inhalation, transdermal, implantable, nanoparticle-based, and connected devices. Evaluated performance, regulatory expectations, and innovation trends used by leading manufacturers. | Helped clients select suitable delivery technologies, compare long-acting and device–drug options, and understand emerging platforms such as mRNA systems and smart injectors. Supported product planning for chronic disease and biologics-focused treatments. |
| Company Information | Profiled major global and Europe drug-delivery players, covering portfolios, platforms, partnerships, manufacturing, and R&D efforts. Analyzed strengths in biologic and sustained-release delivery. | Enabled clients to gauge competitor capabilities, assess supplier fit for injectables and device partners, and identify collaboration or outsourcing opportunities. Strengthened long-term strategy in biologics and advanced delivery solutions. |
| Geographic Analysis | Examined Europe delivery trends across regulatory systems, biologics expansion, home-care adoption, and manufacturing hubs. Included reimbursement and digital-health ecosystem insights, with optional country-level customization. | Supported strategic market entry by identifying growth areas, localization options, and supply-chain opportunities. Helped clients evaluate patient preferences and emerging demand in home-based and biologics-driven delivery segments across diverse Europe markets. |
RECENT DEVELOPMENTS
- December 2025 : Moderna entered a USD 500 million partnership with Nanexa to enhance long-acting injectable drug delivery technology for mRNA medicines, aiming to improve stability and patient dosing convenience in Europe.
- April 2025 : Roche received approval from the European Commission for its Columvi (glofitamab) injection for relapsed or refractory DLBCL, strengthening Europe’s oncology injectable and antibody-based drug delivery landscape.
- January 2025 : The European Commission approved Johnson & Johnson’s subcutaneous RYBREVANT (amivantamab), improving injectable drug delivery convenience and reducing administration time in advanced lung cancer treatment.
- May 2024 : Novartis took a step to expand its cancer care portfolio through the acquisition of Mariana Oncology, which in turn gives the company access to next-generation radioligand therapies for the most challenging oncology issues.
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation, industry trends, key players, competitive landscape, fundamental market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the extensive use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), European Federation of Pharmaceutical Industries and Associations (EFPIA), American Journal of Drug Delivery and Therapeutics, Center for Drug Delivery and Nanomedicine (CDDN) and Parenteral Drug Association (PDA)] to identify and collect information for the Europe pharmaceutical drug delivery market study. It was used to obtain important information about the key players and market classification & segmentation according to industry trends, to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. Primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the Europe pharmaceutical drug delivery market. Meanwhile, primary sources from the demand side include hospitals, ambulatory surgical centers/clinics, home care settings, and diagnostic centers, among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on industry trends and market dynamics.
To know about the assumptions considered for the study, download the pdf brochureMarket Size Estimation
To estimate the market value, annual revenues were calculated based on the revenue mapping of major product manufacturers and OEMs active in the Europe pharmaceutical drug delivery market. All the major product manufacturers were identified at the regional and/or country level. Revenue mapping for the respective business segments/subsegments was done for the major players. Additionally, the Europe pharmaceutical drug delivery market was split into various segments and subsegments based on:
- List of major players operating in the Europe pharmaceutical drug delivery products market in Europe and/or at the country level
- Product mapping of various pharmaceutical drug delivery manufacturers in Europe and/or at the country level
- Mapping of annual revenue generated by listed major players from Europe pharmaceutical drug delivery (or the nearest reported business unit/product category) in Europe
- Revenue mapping of major players to cover at least 45% of the Europe market share as of 2025
- Extrapolation of the revenue mapping of the listed major players to derive the Europe market value of the respective segments/subsegments
- Summation of the market value of all segments/subsegments to arrive at the Europe pharmaceutical drug delivery market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
Europe Pharmaceutical Drug Delivery Market : Top-Down and Bottom-Up Approach
Data Triangulation
After arriving at the overall size of the Europe pharmaceutical drug delivery market through the above-mentioned methodology, the market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value for key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Market Definition
Drug delivery involves administering a pharmaceutical drug to achieve its intended therapeutic effect. Advances in drug delivery provide various benefits, such as increased ease of use, convenience, and improved patient compliance. Drug delivery technologies are utilized for targeted delivery and controlled release of therapeutic agents.
Key Stakeholders
- Pharmaceutical manufacturing companies
- Original equipment manufacturing companies
- Pharmaceutical product and medical device suppliers and distributors
- Healthcare service providers
- Teaching hospitals and academic medical centers
- Health insurance players
- Government bodies/Municipal corporations
- Regulatory bodies
- Medical research institutes
- Business research and consulting service providers
- Venture capitalists
- Market research and consulting firms
Report Objectives
- To define, describe, and forecast the size of the Europe pharmaceutical drug delivery market based on route of administration, application, care setting, and country
- To forecast the sizes of market segments with respect to the following countries: Germany, the UK, France, Italy, Spain, the Netherlands, Belgium, Sweden, and the Rest of Europe
- To identify and analyze key drivers, restraints, opportunities, and challenges influencing market growth
- To strategically analyze micro markets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze opportunities in the market for key stakeholders and provide details of the competitive landscape
- To profile the key market players and comprehensively analyze their market shares and core competencies
- To track and analyze competitive developments such as mergers and acquisitions, product developments, partnerships, agreements, collaborations, and expansions
Available customizations:
With the given market data, MarketsandMarkets offers customizations per your company’s specific needs. The following customization options are available for the Europe pharmaceutical drug delivery market report.
Product Analysis
- Product Matrix, which gives a detailed comparison of the product portfolios of the top five market players
Regional Analysis
- Further breakdown of the Rest of Europe pharmaceutical drug delivery market into Austria, Switzerland, Finland, Poland, and Portugal, among others
Company Information
- Detailed analysis and profiling of additional market players (up to five)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Pharmaceutical Drug Delivery Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Europe Pharmaceutical Drug Delivery Market