
US Pharmaceutical Drug Delivery Market Size, Growth, Share & Trends Analysis
US Pharmaceutical Drug Delivery Market by Route of Administration [Oral (Tablet, Capsule), Injectable, Topical (Cream, Transdermal, Suppositories), Ocular, Nasal, Transmucosal, Implantable], Application (Cancer, Diabetes), Care Setting - Forecast to 2031




OVERVIEW
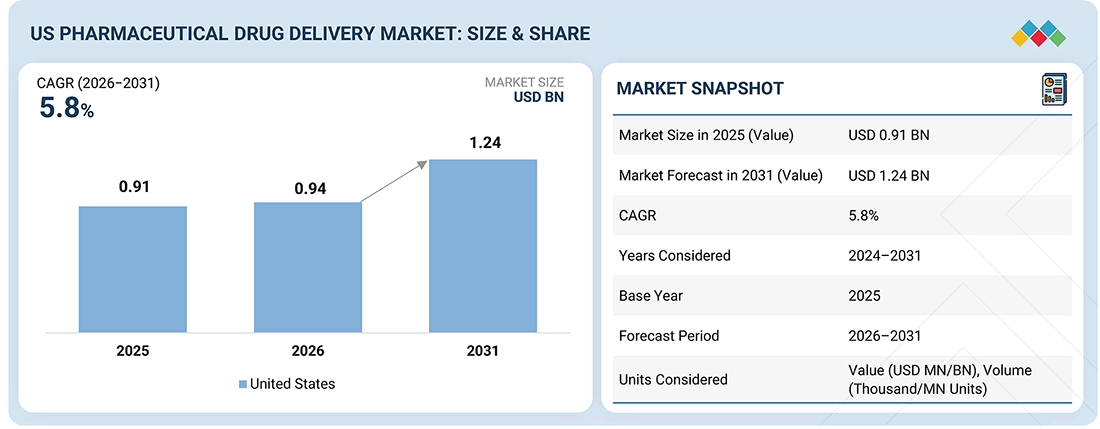
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The US pharmaceutical drug delivery market, valued at US$0.91 billion in 2025, stood at US$0.94 billion in 2026 and is projected to advance at a resilient CAGR of 5.8% from 2026 to 2031, culminating in a forecasted valuation of US$1.24 billion by the end of the period. The market growth trend is fueled by several factors. One of the primary drivers is the quick and extensive rise of biologics and biosimilars, necessitating advanced injectable and large-volume delivery systems. Moreover, the increasing number of chronic diseases, such as cancer, diabetes, and autoimmune disorders, that need self-administered therapies is also contributing to this growth trend. Furthermore, the widespread adoption of home-based and wearable drug delivery systems plays a key role in this process by making it easier for patients to take their medications and thus improving their compliance. Other factors contributing to market growth are the constant development of smart, connected, and sensor-enabled injectors that not only help to expand the use of prefilled syringes for safety and dosing accuracy but also act as a means to increase the use of prefilled syringes.
KEY TAKEAWAYS
-
By Route of AdministrationBy route of administration, the injectable drug delivery segment is expected to register the highest CAGR of 7.1% during the forecast period.
-
By ApplicationBy application, the other applications segment is expected to dominate the market, with a share of 32.5% in 2025.
-
By Care SettingBy care setting, the hospitals segment is expected to dominate the market, with a share of 58.9% in 2025.
-
Competitive Landscape - Key PlayersJohnson & Johnson Services, Inc. (US, Pfizer Inc. (US), F. Hoffmann-La Roche (Switzerland), Becton, Dickinson and Company (US), and Merck & Co., Inc. (US) were identified as the star players in the US pharmaceutical drug delivery market, given their extensive global reach and comprehensive product portfolios.
-
Competitive Landscape - StartupsRani Therapeutics (US), Enable Injections (US), Portal Instruments (US), and Zosano Pharma (US) have distinguished themselves among startups and SMEs due to their specialized veterinary expertise and focused service capabilities.
The pharmaceutical drug delivery market in US is experiencing strong growth due to the surge in complex biologics that require specialized injectable, transdermal, and inhalation delivery platforms, along with a rising shift toward self-administration as patients increasingly manage chronic diseases at home. Demand is further accelerated by advancements in wearable and on-body injectors, safety-engineered prefilled syringes, and digital drug delivery systems, which improve adherence and enable real-time monitoring. Additionally, pharmaceutical manufacturers are investing heavily in novel formulations—such as long-acting injectables, nanoparticle-based systems, and controlled-release technologies—to enhance therapeutic efficacy and patient experience. Regulatory focus on reducing medication errors and improving the safety of device-drug combinations also fuels the adoption of more advanced, user-friendly delivery solutions across healthcare settings.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The massive trends and disruptions, along with the transition from traditional small-molecule drugs to biologics, have started to alter the US pharmaceutical drug delivery market. This change is marked by huge investments in advanced injectables and wearable systems for large-molecule biologics. Such a disruption is accompanied by the surge in connected and sensor-enabled devices aimed at supporting digital therapies and remote monitoring of patients, as well as the increasing acceptance of self-administered solutions, which minimize the need for clinical settings. Additionally, new medical approaches like on-body injectors, microneedle patches, nanoparticle-based, and smart inhalers are changing the face of drug delivery. Nevertheless, supply chain troubles, sterility requirements, and tougher regulations for combination products are compelling pharmaceutical manufacturers to adopt more automated, user-centric, and technology-integrated delivery platforms.
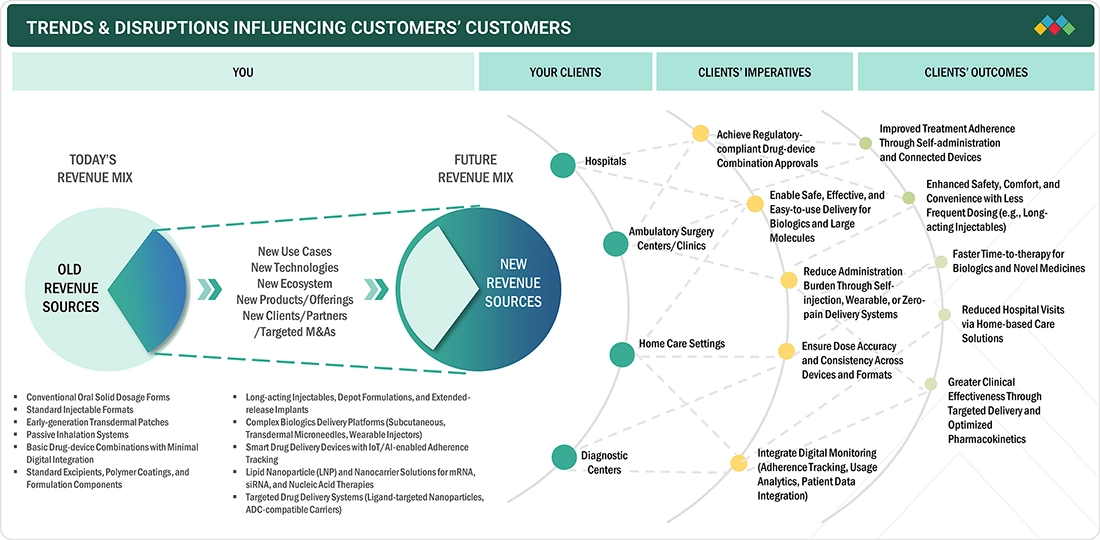
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising demand for biologics and complex therapies

-
Shift toward self-administration and home-based care
Level
-
High development and manufacturing costs
-
Stringent regulatory and compliance requirements
Level
-
Expansion of wearable and connected delivery systems
-
Rising adoption of long-acting and targeted delivery technologies
Level
-
Stringent regulatory environment and complex approval pathways
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising demand for biologics and complex therapies
The increasing need for biologics and complicated therapies is the primary factor boosting the US pharmaceutical drug delivery market. Biologics, which consist of monoclonal antibodies, vaccines, and gene therapies, depend on advanced delivery systems that are capable of preserving the product's characteristics throughout the process, ensuring proper release, and making it more convenient for the patient to comply. With the increasing occurrence of chronic conditions, cancer, and autoimmune diseases, the pharmaceutical industry is moving towards advanced delivery systems like injectables, sustained-release formulations, and even combination devices.
Restraint: High development and manufacturing costs
In the US pharmaceutical drug delivery market, high development and manufacturing costs present a substantial limitation. The entire process of advanced delivery systems, like biologics, nanocarriers, and combination devices, involves extensive R&D, specialized tools, and rigorous quality control, which raises the cost of production. Such high costs can mean longer times for new products to reach the market, lower profit margins, and a reduction in the number of small firms.
Opportunity: Expansion of wearable and connected delivery systems
The rise of wearable and connected drug delivery systems is a major factor in the US pharmaceutical drug delivery market. Smart insulin pumps, connected inhalers, and digital injectors not only enable real-time monitoring but also provide personalized dosing and improved patient adherence. Consequently, they align with the growing trend of home care and chronic disease management, which is receiving increasing attention. By integrating digital health technologies with drug delivery, not only are treatment outcomes improved, but data-driven healthcare solutions are also being developed. This, in turn, is fostering innovation and market growth.
Challenge: Stringent regulatory environment and complex approval pathways
The US pharmaceutical drug delivery market faces significant challenges due to stringent regulatory approvals, which serve as a major obstacle. New delivery systems classified as combination devices, biologics, or novel formulations must meet complex FDA requirements concerning safety, efficacy, and quality. Prolonged approval times, rigorous clinical trials, and changing regulations can delay product launches, increase development costs, and limit the number of new entrants in the market. This situation ultimately restricts innovation within the industry.
US Pharmaceutical Drug Delivery Market: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
J&J acquired Alza Corporation in 2001, inheriting advanced drug-delivery platforms including osmotic oral systems (OROS), transdermal patches, implantable pumps, liposomal systems, and controlled-release technologies. The company continues to invest in combining pharmaceuticals with medical devices across oncology, immunology, neuroscience, and cardiopulmonary areas. | Johnson & Johnson’s diverse delivery technologies enable controlled, sustained, or targeted drug release, thereby improving patient compliance compared to frequent dosing. Its broad coverage across oral, transdermal, implantable, and injectable modalities provides flexibility across multiple therapeutic areas. Integration with its medical device business creates synergies for drug-device combinations, especially for biologics and long-acting therapies. |
 |
Pfizer operates across oral tablets, injectable formulations, and advanced delivery systems, including sustained-release and liposomal formats. It also works on lipid-nanoparticle-based delivery technologies, including mRNA therapies. Legacy collaborations with Alza supported the development of controlled-release products for chronic therapies. | Pfizer’s extensive delivery capabilities offer flexibility in delivering both small-molecule drugs and complex therapies. Controlled-release formulations improve chronic disease management by enhancing adherence and therapeutic consistency. The company’s strong R&D and commercial scale allow efficient adoption of emerging delivery technologies, such as lipid-nanoparticle systems for advanced therapies. |
 |
Roche focuses on specialty delivery platforms, including implantable or port-based systems for ocular or sustained-release therapies, antibody-drug conjugates, and injectable biologics for oncology, immunology, and ophthalmology. The company also maintains a strong presence in the injectable drug delivery market. | Roche’s delivery technologies support sustained or localized administration, reducing the frequency of dosing and improving compliance. Its expertise in ADCs and biologics enables the targeting of complex diseases, providing a competitive advantage as biologic therapies continue to grow. Strength in specialty therapeutics supports high-value niche markets, where patients benefit from precise and convenient treatment options. |
 |
Novartis is expanding its drug-device and advanced-delivery pipeline with inhalation systems, injectable biologics, and subcutaneous administration technologies, such as microglassification. It is building dedicated manufacturing infrastructure in the US to support radioligand therapies and other advanced treatments. | Novartis emphasizes patient-friendly administration routes that enhance convenience and facilitate self-administration, thereby improving adherence to biologics and long-term therapies. Its ability to deliver complex therapies, including radioligand and gene- or cell-based treatments, supports growth in precision medicine. The US manufacturing footprint enhances supply reliability and facilitates efficient navigation of regulatory requirements. |
 |
Merck maintains a broad portfolio spanning vaccines, oncology, antivirals, and other therapeutic areas, which requires multiple delivery formats, including oral, injectable, and advanced nano-enabled systems for complex molecules. It is positioned as a leading provider in the injectable and biologics drug delivery market. | Merck’s extensive portfolio provides flexibility to tailor delivery methods across multiple therapeutic areas. Its scale and experience support the adoption of advanced delivery formats as demand for biologics, vaccines, and complex therapies grows. A diversified therapeutic footprint reduces reliance on any single modality, positioning the company to adapt effectively to emerging delivery technologies. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The present-day US pharmaceutical drug delivery ecosystem is portrayed by a network of the largest and most established drug companies, specialized delivery device firms, regulators, and healthcare providers. The network is, however, maturing and evolving all the time as it tries to accommodate the rising demand for medicines that are more convenient, effective, and patient-centric. The supply side of the equation is mainly characterized by strong R&D investments with biologics, nanotechnology, and controlled-release technologies as the main areas. The Food and Drug Administration (FDA) in the US supports the introduction of new delivery systems through regulatory measures. This support responds to a growing demand driven by the high prevalence of chronic diseases such as diabetes, cancer, and heart disease, as well as an increasing elderly population. These factors contribute to a larger market for injectables, inhalers, implantables, self-administered devices, and long-acting formulations. Additionally, the shift from hospital-based dosing to ambulatory care and home administration, facilitated by the rise of smart injectors and connected drug delivery devices, highlights this change in demand.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
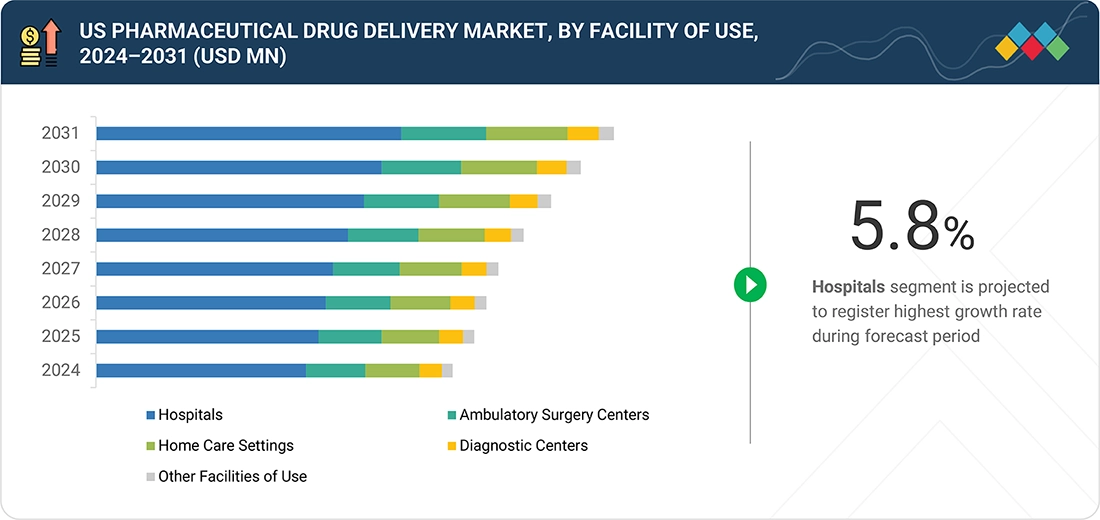
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
US Pharmaceutical Drug Delivery Market, By Route of Administration
In 2025, the injectable drug delivery devices segment is expected to hold the largest share of the US pharmaceutical drug delivery market. The market will be significantly influenced by the rise in biologics, monoclonal antibodies, vaccines, and other large-molecule therapies that require parenteral administration. The rising occurrence of chronic diseases such as cancer, diabetes, autoimmune disorders, and cardiovascular conditions creates the need for more frequent, reliable, and precise dosing, which injectables can deliver more efficiently than oral routes. Additionally, there is a strong preference among patients and healthcare providers for self-administration tools such as autoinjectors, prefilled syringes, and pen injectors, which improve convenience, reduce clinic visits, and support home care. Safety-engineered devices, needle-free injectors, and on-body delivery systems are some technological advances that drive market growth by improving safety, usability, and treatment adherence.
US Pharmaceutical Drug Delivery Market, By Application
In 2025, the infectious diseases application segment is expected to hold the largest share of the US pharmaceutical drug delivery market. Market growth can be attributed to the growing number of viral and bacterial outbreaks, the high prevalence of hospital-acquired infections, and the rise in antimicrobial resistance, which requires stronger formulations and better delivery routes. The growing adoption of immunocompromising treatments (e.g., cancer therapy and organ transplants) also contributes to the demand for more sophisticated injectable, vaccine, and targeted delivery systems, as they have the potential to make patients more vulnerable to infections. Furthermore, factors such as globalization, increased population movement, and an aging population exacerbate the infection scenario. This accelerates the demand for drug delivery solutions that are efficient, quick-acting, and user-friendly.
US Pharmaceutical Drug Delivery Market, By Care Setting
In 2025, the US pharmaceutical drug delivery market is going to see a rise in the hospitals segment due to the increasing number of both chronic and acute patients who need to undergo complex therapies that can only be administered through controlled, injectable, or device-assisted systems. The growing use of biologics, oncology drugs, radiopharmaceuticals, and advanced infusion-based therapies is driving more patients to seek care in hospital settings. Hospitals are equipped with specialized staff, cold-chain infrastructure, and high-precision delivery technologies essential for these treatments. They also play a crucial role in providing emergency care, performing surgeries, and administering inpatient treatments that often involve fast-acting or high-risk drug delivery methods. Additionally, the rising trend of utilizing connected infusion pumps, smart injectors, and combination devices within hospital systems enhances safety and treatment efficiency, further increasing the demand for hospitals in the drug delivery ecosystem.
US Pharmaceutical Drug Delivery Market: COMPANY EVALUATION MATRIX
In the US pharmacy drug delivery market matrix, the star players are Johnson & Johnson, Pfizer, Merck, Roche, and Novartis. These companies greatly benefit from their R&D expertise, diverse delivery options, and strong positions in biologics, oncology, vaccines, and therapies for chronic diseases. Their position of dominance is backed by especially reliable technologies like sustained-release systems, implantable devices, smart injectors, lipid-nanoparticle platforms, and drug combinations. These players also benefit from large-scale production, regulatory know-how, and tech support. On the other hand, the industry’s emerging leaders include Boehringer Ingelheim International GmbH. These players are making significant progress by focusing on specific delivery methods. These methods comprise microneedles, wearables, nanoparticle systems, implants for ocular delivery, and innovations for biologic delivery through subcutaneous. They are steadily gaining popularity due to their extremely diverse, patient-focused products that correspond with the trends of home care and self-administration. In addition to major suppliers, niche technology providers and mid-sized firms are expanding their expertise in inhalation systems, skin patches, depot injections, and connected digital delivery devices. The combination of resources and capabilities from established players, along with the innovative, technology-driven advantages of new entrants, is transforming the US pharmaceutical drug delivery market into a highly competitive and rapidly growing sector.
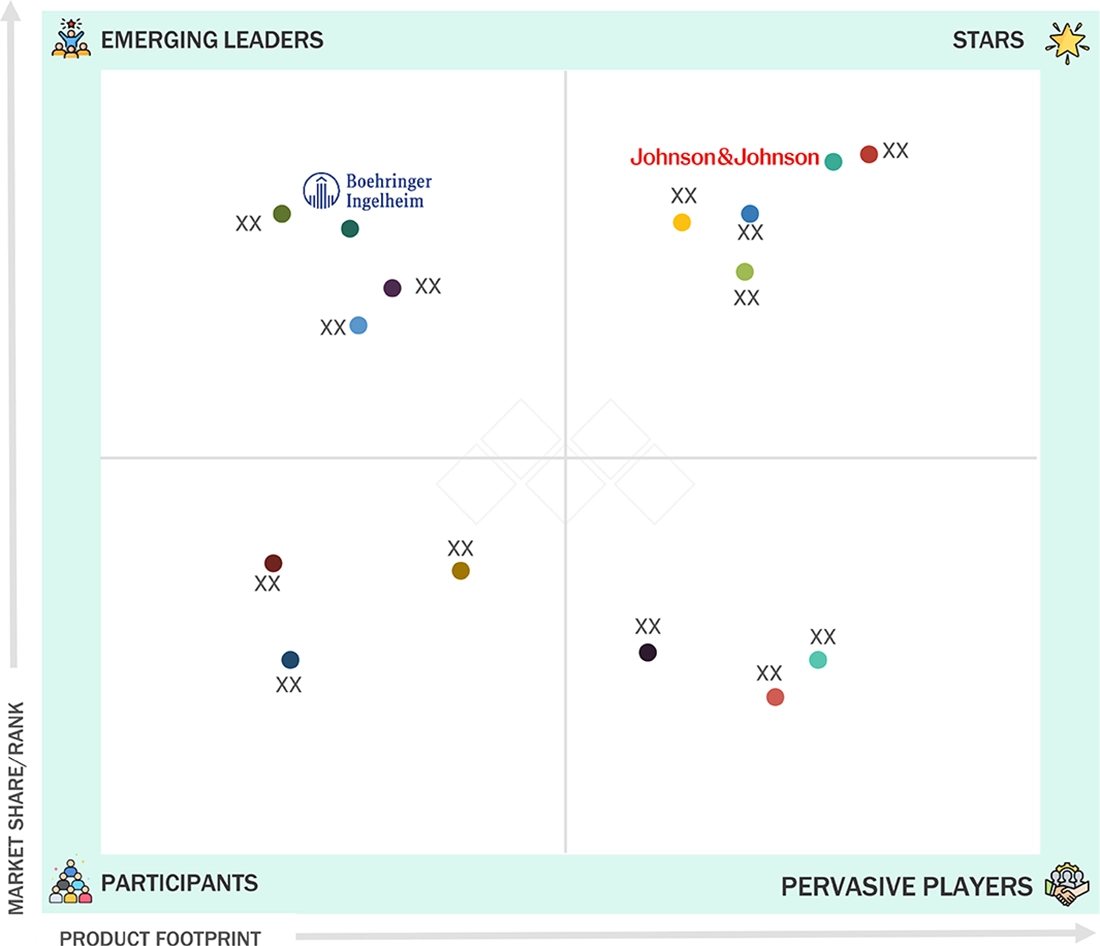
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Johnson & Johnson Services, Inc. (US)
- Pfizer Inc. (US)
- F. Hoffmann-La Roche (Switzerland)
- Becton, Dickinson and Company (US)
- Merck & Co., Inc. (US)
- Amgen, Inc. (US)
- AbbVie Inc. (US)
- Gilead Sciences, Inc. (US)
- Eli Lilly and Company (US),
- Bristol-Myers Squibb (US)
- Biogen (US)
- Baxter International (US)
- West Pharmaceutical Services (US)
- Catalent Pharma Solutions (US),
- Novartis (Switzerland)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2025 (Value) | USD 0.91 Billion |
| Market Forecast in 2031 (Value) | USD 1.24 Billion |
| Growth Rate | CAGR of 5.8% from 2026–2031 |
| Years Considered | 2024–2031 |
| Base Year | 2025 |
| Forecast Period | 2026–2031 |
| Units Considered | Value (USD Million/Billion), Volume (Thousand/Million Units) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Country Covered | US |
| Parent & Related Segment Reports |
Pharmaceutical Drug Delivery Market Europe Pharmaceutical Drug Delivery Market APAC Pharmaceutical Drug Delivery Market |
WHAT IS IN IT FOR YOU: US Pharmaceutical Drug Delivery Market REPORT CONTENT GUIDE
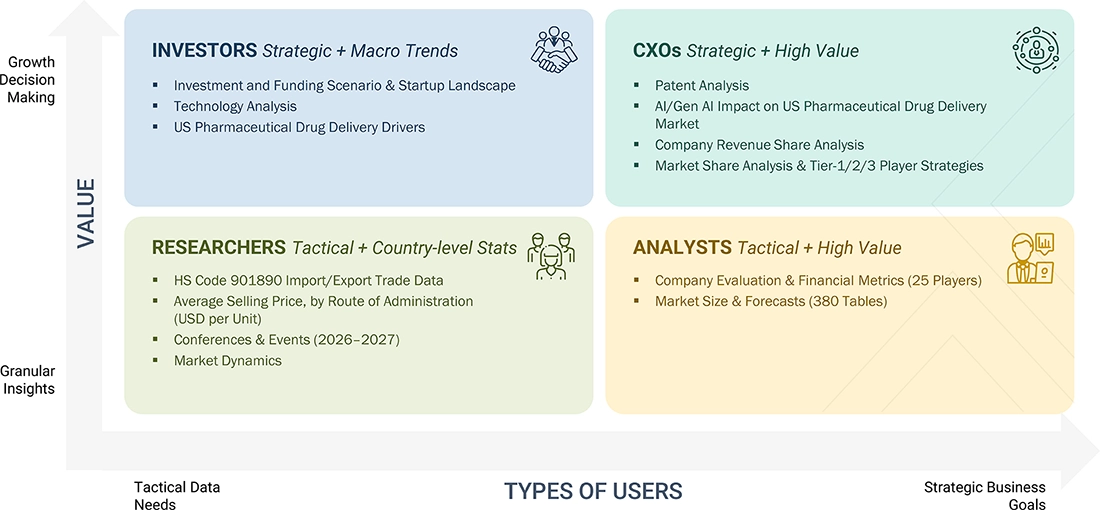
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis |
|
|
| Company Information |
|
|
| Geographic Analysis |
|
|
RECENT DEVELOPMENTS
- December 2024 : Becton Dickinson (BD) (US) expanded its injectable drug delivery portfolio with the launch of the BD EffiFlow Advanced Prefillable Syringe Platform, designed to enhance biologics compatibility, reduce extrusion force, and improve safety in high-viscosity drug administration. This portfolio expansion strengthens BD’s leadership in prefillable syringes and supports growing demand for self-injection solutions across the US biologics and specialty drug markets.
- November 2024 : West Pharmaceutical Services (US) introduced its next-generation NovaPure 3.0 Components, featuring improved elastomer purity, enhanced container-closure integrity, and optimized performance for sensitive biologics and mRNA formulations. This launch underscores West’s focus on high-performance containment and delivery systems, supporting its expansion into advanced sterile drug packaging for the US pharmaceutical sector.
- April 2024 : Baxter International (BAX) expanded its pharmaceuticals/injectables portfolio with a set of new injectable product launches in the US, reinforcing its offering for hospitals and specialty injectable therapies and underlining Baxter’s continued role in parenteral product supply chains. Baxter reported full-year 2024 continuing operations sales of approximately USD 10.64 billion.
Table of Contents

Methodology
This study involved the extensive use of both primary and secondary sources. The research process involved the study of various factors affecting the industry to identify the segmentation types, industry trends, key players, competitive landscape, fundamental market dynamics, and key player strategies.
Secondary Research
The secondary research process involves the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, SEC filings of companies and publications from government sources [such as National Institutes of Health (NIH), US FDA, US Census Bureau, World Health Organization (WHO), Centers for Disease Control and Prevention (CDC), European Federation of Pharmaceutical Industries and Associations (EFPIA), American Journal of Drug Delivery and Therapeutics, Center for Drug Delivery and Nanomedicine (CDDN) and Parenteral Drug Association (PDA) were referred to identify and collect information for the US pharmaceutical drug delivery market study. It was also used to obtain important information about the key players and market classification & segmentation according to industry trends to the bottom-most level and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations in the pharmaceutical drug delivery market. The primary sources from the demand side include hospitals, ambulatory surgical centers/clinics, home care setting, diagnostic centers among others. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends & key market dynamics.
Market Estimation Methodology
For the US market value, annual revenues were calculated based on the revenue mapping of major product manufacturers and OEMs active in the US pharmaceutical drug delivery market. All the major product manufacturers were identified at the US and/or country/regional level. Revenue mapping for the respective business segments/sub-segments was done for the major players (who contribute at least 45-48% of the market share at the US level). Also, the US pharmaceutical drug delivery market was split into various segments and sub-segments based on:
- List of major players operating in the pharmaceutical drug delivery products market at the regional and/or country level
- Product mapping of various pharmaceutical drug delivery manufacturers at the regional and/or country level
- Mapping of annual revenue generated by listed major players from pharmaceutical drug delivery (or the nearest reported business unit/product category)
- Revenue mapping of major players to cover at least 45% of the uS market share as of 2023
- Extrapolation of the revenue mapping of the listed major players to derive the US market value of the respective segments/subsegments
- Summation of the market value of all segments/subsegments to arrive at the US point-of-care diagnostics market
The above-mentioned data was consolidated and added with detailed inputs and analysis from MarketsandMarkets and presented in this report.
Market Size Estimation: US Pharmaceutical Drug Delivery Market Based On Revenue Mapping Methodology

To know about the assumptions considered for the study, Request for Free Sample Report
Data Triangulation
After arriving at the overall size of the Us Pharmaceutical drug delivery market through the above-mentioned methodology, this market was split into several segments and subsegments. The data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact market value data for the key segments and subsegments. The extrapolated market data was triangulated by studying various macro indicators and regional trends from both demand- and supply-side participants.
Market Definition
Drug delivery is a method or process of administration of a pharmaceutical drug to safely attain its desired therapeutic effect. Advancements in drug delivery offer several benefits, such as ease of use, convenience, and patient compliance. Drug delivery technologies are used for the targeted delivery and/or controlled release of therapeutic agents.
Key Market Stakeholders
- Pharmaceutical manufacturing companies
- Original equipment manufacturing companies
- Suppliers and distributors of pharmaceutical products and medical devices
- Healthcare service providers
- Teaching hospitals and academic medical centers
- Health insurance players
- Government bodies/municipal corporations
- Regulatory bodies
- Medical research institutes
- Business research and consulting service providers
- Venture capitalists
- Market research and consulting firms
Report Objectives
- To define, describe, and forecast the US pharmaceutical drug delivery market on the basis of US pharmaceutical drug delivery product, route of administration, application, facility of use, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets1 with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for key stakeholders and provide details of the competitive landscape for major market leaders
- To forecast the size of the market segments with respect to five main regions, namely, North America (the US and Canada), Europe (Germany, the UK, France, Italy, Spain, and Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, and Rest of Asia Pacific), Latin America (Brazil, Mexico, and Rest of Latin America), and the Middle East & Africa
- To profile the key market players and comprehensively analyze their market shares and core competencies2
- To track and analyze competitive developments such as mergers and acquisitions, new product developments, partnerships, agreements, collaborations, and expansions in the US pharmaceutical drug delivery market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per your company’s specific needs. The following customization options are available for the US pharmaceutical drug delivery market report.
Product Analysis
- Product Matrix, which gives a detailed comparison of the product portfolios of the top five US players.
Company Information
- Detailed analysis and profiling of additional market players (up to 5 players)
Geographic Analysis
- Further breakdown of the Rest of Europe pharmaceutical drug delivery market into Belgium, Austria, the Netherlands, Switzerland, Austria, Finland, Sweden, Poland, and Portugal, among other
- Further breakdown of the Rest of Asia Pacific pharmaceutical drug delivery market into New Zealand, Vietnam, Philippines, Singapore, Malaysia, Thailand, and Indonesia, among other countries
- Further breakdown of the Rest of Latin America pharmaceutical drug delivery market into Argentina, and Colombia, among other countries.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the US Pharmaceutical Drug Delivery Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in US Pharmaceutical Drug Delivery Market