
Asia Pacific Pharmaceutical Filtration Market Size, Growth, Share & Trends Analysis
Asia Pacific Pharmaceutical Filtration Market by Product [Membrane Filter, Depth Filter, Virus Filter, Air Filter, Assemblies, System (Single-use)], Technique (Ultrafiltration), Type (Sterile), Application (API, Protein), Scale, End User-Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
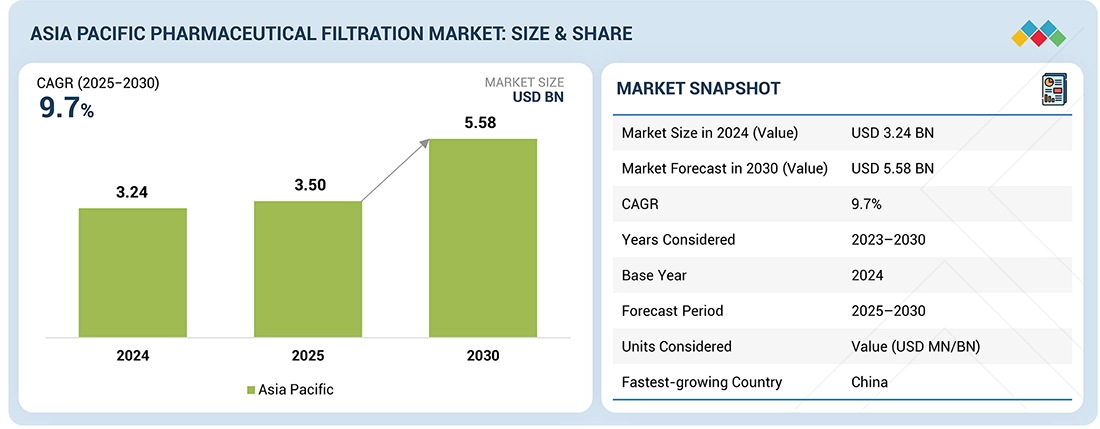
The Asia Pacific pharmaceutical filtration market, valued at USD 3.24 billion in 2024, stood at USD 3.50 billion in 2025 and is projected to advance at a resilient CAGR of 9.7% from 2024 to 2030, culminating in a forecasted valuation of USD 5.58 billion by the end of the period. Pharmaceutical filtration is a process wherein solid and semi-solid particles present in a suspension are separated from a liquid or gas by employing membrane filters, such as filter sheets, cartridges & capsules, papers, depth filters, and others. Growth in this market can primarily be attributed to the rapid expansion of biologics & vaccine manufacturing in the Asia Pacific region.
KEY TAKEAWAYS
-
BY COUNTRYBy country, China accounted for the largest share of 34.4% of the Asia Pacific pharmaceutical filtration market in 2024.
-
BY PRODUCTBy product , the consumables segment accounted for the largest share of 83.6% of the market in 2024.
-
BY TECHNIQUEBy technique, the microfiltration segment is projected to grow at the fastest rate from 2025 to 2030.
-
BY TYPEBy type, sterile preparations dominate the Asia Pacific pharmaceutical filtration market.
-
BY APPLICATIONBy application, the final product processing segment is expected to dominate the market.
-
BY SCALE OF OPERATIONBy scale of operation, the manufacturing scale segment is expected to register the highest growth rate during the forecast period.
-
BY END USERBy end user, the pharmaceutical & biopharmaceutical companies segment is expected to dominate the market.
-
COMPETITIVE LANDSCAPEAsahi Kasei Corporation (Japan), Advanced Microdevices Pvt. Ltd. (MDI) (India), and Depth Filtration Technologies (India) were identified as the Star players in the Asia Pacific pharmaceutical filtration market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPEShanghai Junyi Filtration Equipment Co., Ltd. (China) and Osmoteck Membranes Pvt. Ltd. (India) have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The pharmaceutical filtration market in Asia Pacific is experiencing growth, driven by quality regulations, rising demand for sterile drug production, and the increasing adoption of biologic therapies. Advanced filtration technologies enable safer and more reliable manufacturing of vaccines, injectable medicines, and personalized treatments. As manufacturers increasingly prioritize contamination control and regulatory compliance, filtration solutions are becoming a vital component for maintaining pharmaceutical quality and fostering innovation.
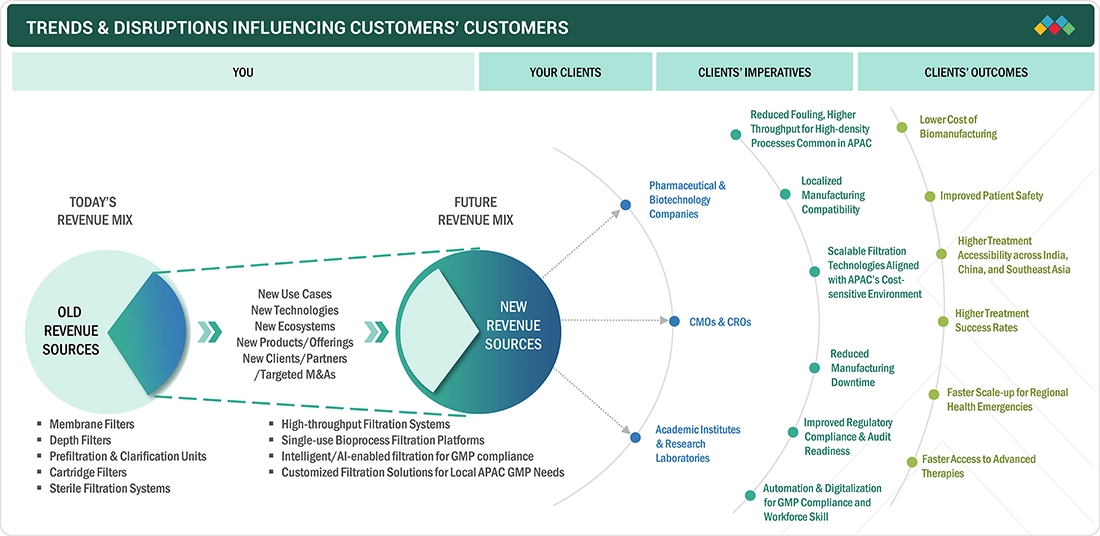
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The Asia Pacific pharmaceutical filtration market is being reshaped by rapidly evolving industry, regulatory, and therapeutic trends, with major shifts expected to deepen over the forecast period. Similar to North America, growth is driven by the rising production of biologics and biosimilars, the wider adoption of single-use bioprocessing systems, and the increasing demand for sterile processing to ensure high product purity and robust contamination control. The accelerated development of advanced therapies, including cell & gene therapies, mRNA vaccines, and recombinant proteins, is further boosting the demand for high-performance filtration technologies across Asia Pacific biomanufacturers and CDMOs, where heightened focus on contamination control and compliance with national authority requirements is making filtration platforms a core component of quality assurance and a key engine of technological innovation from upstream through fill-finish operations.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rapid expansion of biologics & vaccine manufacturing in Asia Pacific region

-
Growing CDMO/CMO footprint
Level
-
Price sensitivity & competitive sourcing
Level
-
Localization of membrane & consumables manufacturing
Level
-
Intense local & global competition
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rapid expansion of biologics & vaccine manufacturing in Asia Pacific region
The Asia Pacific is witnessing accelerated growth in monoclonal antibody, recombinant protein, and vaccine production, driven by strong government funding, domestic biologics initiatives, and rising therapeutic demand. As more facilities scale up upstream and downstream bioprocessing, the region’s need for high-performance sterile, depth, and virus filtration systems continues to grow, boosting market demand across all manufacturing phases.
Restraint: Price sensitivity & competitive sourcing
Many Asia Pacific manufacturers operate under cost-sensitive procurement models that prioritize low-cost consumables and competitive bidding. This environment challenges premium filtration suppliers, slowing the adoption of advanced, higher-priced membrane and single-use technologies despite their performance benefits.
Opportunity: Localization of membrane & consumables manufacturing
Countries such as China, India, South Korea, and Singapore are investing in local membrane production, filter assembly, and bioprocess consumables manufacturing to reduce dependence on imports. This localization enhances supply chain resilience, reduces lead times, and fosters strong opportunities for partnerships, technology transfer, and region-specific product customization.
Challenge: Intense local & global competition
In the Asia Pacific region, manufacturers face pressure to strengthen sterility assurance, control leachables, and improve sustainability, while navigating a rapidly evolving and highly fragmented regulatory landscape across China, India, Japan, Korea, ASEAN, and other markets. The challenge is not only to meet EMA and ICH expectations that global companies treat as a baseline, but also to translate those high standards into practical filtration strategies that work consistently across very different facilities, supply chains, and regulatory cultures in Asia Pacific.
ASIA PACIFIC PHARMACEUTICAL FILTRATION MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Provides membrane cartridges, virus-removal filters, and single-use filtration systems used for large-scale clarification, sterile filtration, and virus safety steps in commercial biologics and plasma-derived product facilities across the Asia Pacific. | Improves viral safety and product purity at manufacturing scale, supports robust, high-throughput processing, and helps regional biomanufacturers meet stringent global regulatory expectations for biologics export. |
 |
Supplies a wide portfolio of membrane filters, capsule filters, and cartridge systems deployed for bulk solution filtration, buffer and media preparation, and final sterile filtration in Indian and Asia Pacific injectable, vaccine and biosimilar plants. | Offers cost-effective, locally available filtration options that enhance operational reliability, reduce lead times and supply risk, and support scale-up of regional manufacturing for both domestic and contract production. |
 |
Delivers syringe and capsule filters, PTFE and PVDF membrane cartridges, and custom assemblies used in pilot-to-manufacturing transitions, utility filtration, and smaller-volume commercial lines for specialty biologics and generics. | Enables flexible, stepwise expansion from development to full-scale production, helps optimize filtration costs for mid-sized manufacturers, and improves contamination control in a wide range of Asia Pacific pharmaceutical facilities. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Asia Pacific pharmaceutical filtration ecosystem comprises a set of closely knit stakeholders, including membrane & equipment manufacturers, system integrators, biopharmaceutical manufacturers, CMOs/CDMOs, distributors, and end users. Biopharma companies and CMOs apply these technologies across upstream, downstream, and fill-finish operations to ensure sterility, product purity, and regulatory compliance. Engineering partners support validation, scale-up, and process optimization, while distributors ensure predictable delivery to manufacturing sites around the world. This integrated ecosystem enhances process efficiency and enriches contamination control, while also supporting the growing production of biologics, vaccines, and advanced therapies.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
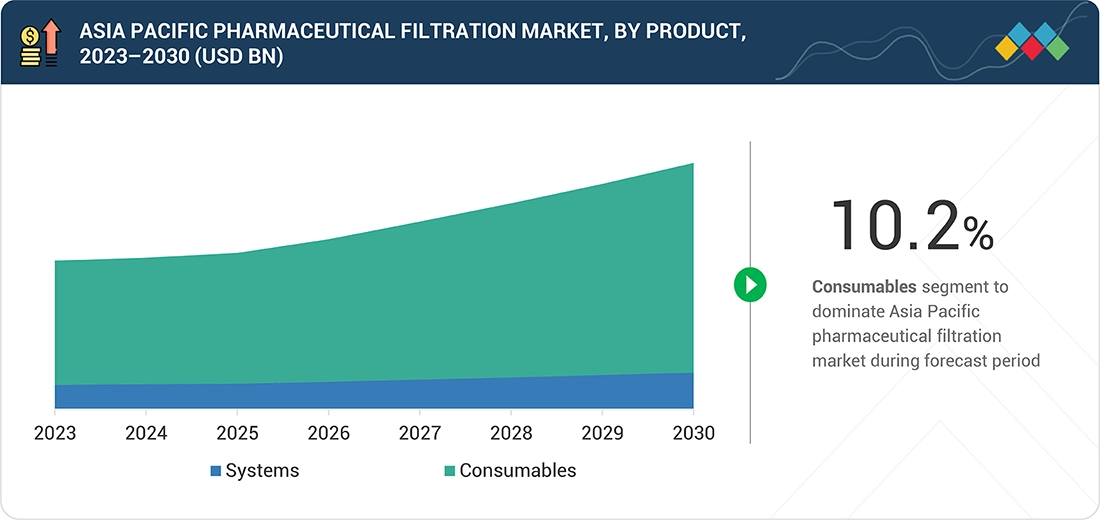
Asia Pacific Pharmaceutical Filtration Market, By Product
In 2024, consumables held the largest share of the Asia Pacific pharmaceutical filtration market, as cartridges, capsules, and single-use filters are used daily and replaced frequently in regional biologics and vaccine plants.
Asia Pacific Pharmaceutical Filtration Market, By Technique
In 2024, microfiltration was the most widely adopted technique, driven by its strong capability to remove bacteria and particulates from high-volume pharmaceutical liquids manufactured across the Asia Pacific.
Asia Pacific Pharmaceutical Filtration Market, By Type
Sterile filtration led the Asia Pacific market in 2024, reflecting its essential role in producing injectable biologics, vaccines, and parenteral nutrition products across the region. The need for complete microorganism removal to meet export-grade GMP standards and protect patient safety underpins its continued dominance.?
Asia Pacific Pharmaceutical Filtration Market, By Application
In 2024, final product processing emerged as the largest application area, as filtration at this stage is vital for ensuring the necessary sterility and purity standards. Its importance in influencing product safety and meeting regulatory requirements makes it a key contributor to market demand.
Asia Pacific Pharmaceutical Filtration Market, By Scale of Operation
Large-scale manufacturing operations held the major share in 2024, supported by rapidly expanding high-volume biologics and vaccine plants in China, India, and South Korea that depend on robust, continuous filtration to maintain consistent quality.
Asia Pacific Pharmaceutical Filtration Market, By End User
Pharmaceutical & biopharmaceutical companies were the leading end-user group in 2024, as their intensive use of filtration technologies for commercial-scale sterile drug production drives most of the region’s filtration demand.
REGION
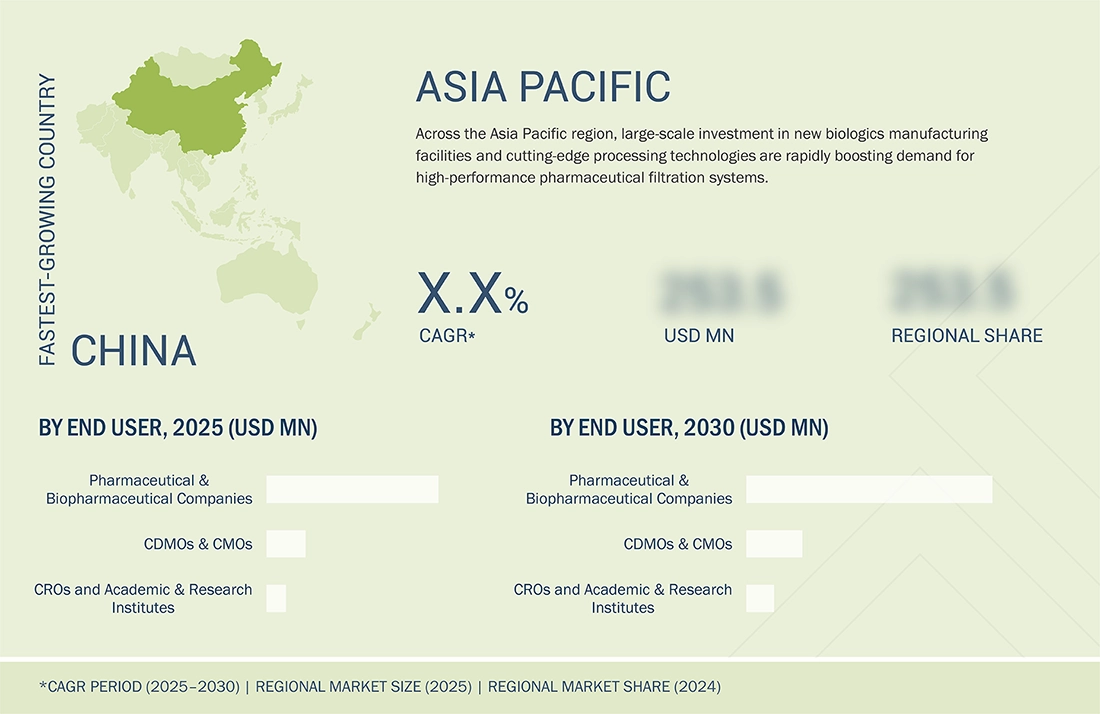
China to be fastest-growing country in market during forecast period
China is one of the fastest-growing markets for manufacturing-scale pharmaceutical filtration, supported by the rapid expansion of biologics and vaccine manufacturing capacity. Growing pipelines in biosimilars and novel biologics, together with heavy investments in contract development and manufacturing, also fuel market growth.
ASIA PACIFIC PHARMACEUTICAL FILTRATION MARKET: COMPANY EVALUATION MATRIX
In the Asia Pacific pharmaceutical filtration market matrix, Asahi Kasei Corporation (Star Player) holds a leading position with an extensive portfolio of high-efficiency filters for sterile and non-sterile applications, a global reach, and robust support across manufacturing and research settings. This leadership has driven widespread adoption among biopharma companies, contract manufacturers, and hospital networks. Depth Filtration Technologies (Emerging Leader) is rapidly gaining traction, owing to its innovative membrane technologies, flexible product design, and expanding presence in precision therapeutic areas. Meanwhile, specialist companies focusing on niche filtration needs and agile manufacturing practices show significant growth potential, with strategic advancements positioning them as future contenders in the leaders’ quadrant.
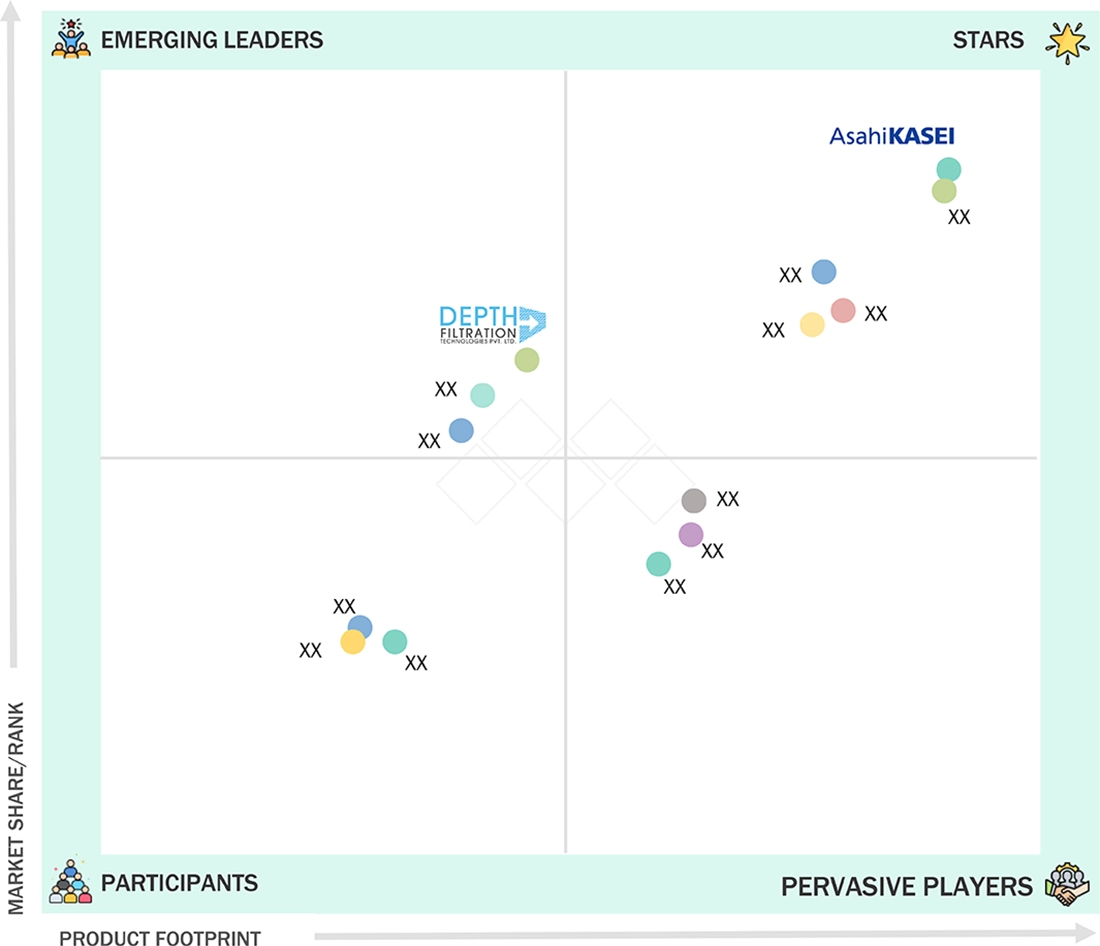
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Asahi Kasei Corporation (Japan)
- Advanced Microdevices Pvt. Ltd. (MdI) (India)
- Axiva Sichem Pvt. Ltd. (India)
- Brother Filtration (China)
- Depth Filtration Technologies Pvt. Ltd.(India)
- KEL Filters (India)
- Osmotech Membranes Pvt. Ltd. (India)
- Star Filter Industry (India)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 3.24 Billion |
| Market Forecast in 2030 (Value) | USD 5.58 Billion |
| Growth Rate | CAGR of 9.7 % from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | China, Japan, India, South Korea, Australia, Rest of Asia Pacific |
| Parent & Related Segment Reports |
Pharmaceutical Filtration Market North America Pharmaceutical Filtration Market Europe Pharmaceutical Filtration Market Pharmaceutical Filtration Consumables Market Pharmaceutical Microfiltration Market |
WHAT IS IN IT FOR YOU: ASIA PACIFIC PHARMACEUTICAL FILTRATION MARKET REPORT CONTENT GUIDE
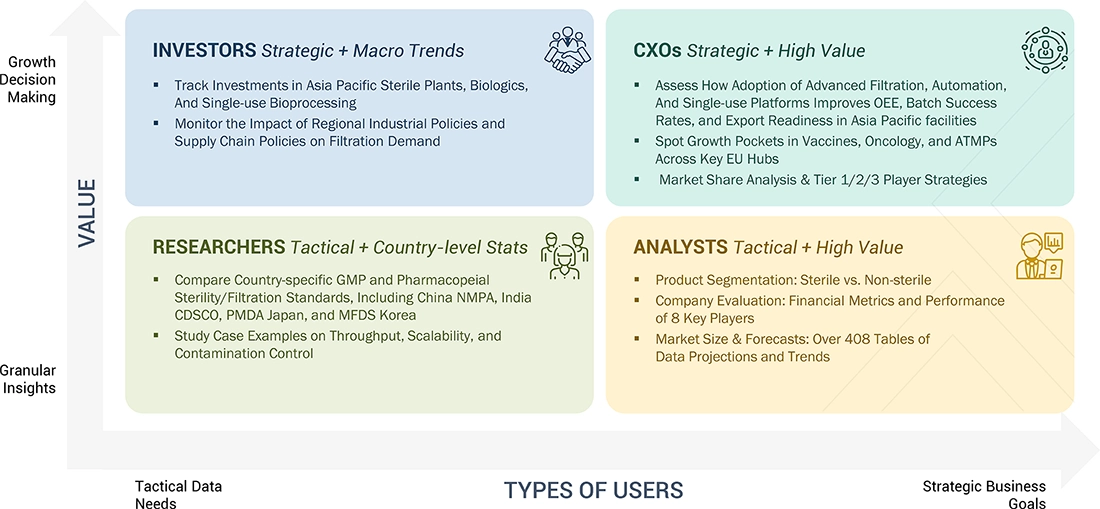
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Filtration Technology Insights (Asia Pacific) | Comprehensive analysis of Asia Pacific microfiltration, ultrafiltration, nanofiltration, sterile, and virus-retentive technologies, comparing efficiency, scalability, cost of ownership, local supply chains, and compliance with regional GMP and export regulations.? | Helps filtration suppliers tailor manufacturing-scale portfolios to differing regulatory environments and plant demands across China, India, South Korea, Japan, Singapore, and other Asia Pacific markets.? |
| Innovation and R&D Trends | Assessment of emerging filtration innovations such as high-throughput single-use systems, continuous bioprocessing, closed sterile manifolds, and PAT-enabled real-time monitoring, linked with analysis of regional R&D funding, partnerships, and patent activity.? | Guides strategic R&D and CAPEX decisions by highlighting high-growth technology niches and mapping how Asia Pacific innovation clusters will shape future manufacturing-scale filtration demand.? |
| Unmet Needs | Identification of filtration bottlenecks in high-titer biologics, vaccines, and cell & gene therapy manufacturing, focusing on scale-up issues, single-use supply security, and country-specific GMP requirements across the Asia Pacific.? | Highlights opportunities for advanced systems and specialized services around contamination control, extractables and leachables management, and validation support tailored to regional manufacturing sites. |
RECENT DEVELOPMENTS
- May 2023 : Asahi Kasei introduced new Microza depth filter membranes designed for applications such as pharmaceutical and biotechnology processing, thereby expanding its depth filtration portfolio for high-capacity liquid clarification and impurity removal.
- May 2024 : Asahi Kasei Medical completed its third assembly plant for Planova virus removal filters in Nobeoka, Japan, boosting its production capacity for hollow-fiber depth-like virus filters used in biotherapeutic manufacturing and strengthening global supply reliability.
Table of Contents

Methodology
This research study extensively used secondary sources, directories, and databases to identify and collect valuable information to analyze the Asia Pacific Pharmaceutical Filtration Market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to gather and verify critical qualitative and quantitative information and assess the market's growth prospects. The market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the Asia Pacific Pharmaceutical Filtration Market. The secondary sources used for this study include the American Membrane Technology Associations (AMTA), European Membrane Society (EMS), American Association of Pharmaceutical Scientists (AAPS), Pharmaceutical Research and Manufacturers of America (PhRMA), National Center for Biotechnology Information (NCBI), Parenteral Drug Association (PDA), Food and Drug Administration (FDA), European Medicines Agency (EMA), Health Canada, National Institutes of Health (NIH), World Health Organization (WHO), Indian Pharmaceutical Association (IPA), International Society for Pharmaceutical Engineering (ISPE), BioProcess International Magazine, BioPharm International, Journal of Bioprocessing and Biotechniques, BioPlan Associates, ScienceDirect, and Factiva, research journals; corporate filings such as annual reports, SEC filings, investor presentations, and financial statements; press releases; trade, business, professional associations and among others. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
Following an initial assessment of the Asia Pacific Pharmaceutical Filtration Market landscape through secondary research, comprehensive primary research was undertaken. This involved conducting in-depth interviews with market experts from the demand side, including stakeholders from pharmaceutical and biotechnology firms, CROs, CMOs, and academic and research institutions. Additionally, interviews were held with key supply-side participants, such as C-suite and senior executives, product managers, and marketing and sales leaders from prominent manufacturers, distributors, and channel partners.
The research covered Asia Pacific, wherein approximately 70% of the primary interviews were conducted with supply-side participants, while 30% involved demand-side experts. Data collection methods included structured questionnaires, email correspondence, online surveys, personal interviews, and telephonic discussions to understand the market dynamics comprehensively.
Market Size Estimation
Both bottom-up and top-down approaches were used to estimate and validate the total size of the Asia Pacific Pharmaceutical Filtration Market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
After arriving at the market size from the estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Pharmaceutical Filtration is a process wherein solid and semi-solid particles present in a suspension are separated from a liquid or gas by employing membrane filters, such as filter sheets, cartridges and capsules, papers, depth filters, and others. These filters retain solids, thus allowing liquids to pass through in all pharmaceutical and biopharmaceutical development and manufacturing processes.
Stakeholders
- Filter Manufacturers, Vendors, and Distributors
- Academic and Government Research Institutes
- Pharmaceutical & Biotechnology Companies
- Life Science Companies
- Venture Capitalists and Investors
- Government Organizations
- Private Research Firms
- Research & Development (R&D) companies
- Contract Research Organizations (CROs)
- Contract Development and Manufacturing Organizations (CDMOs)
- Market Research and Consulting Firms
Report Objectives
- To define, describe, and forecast the Asia Pacific Pharmaceutical Filtration Market based on product, technique, type, scale of operation, application, end user, and region
- To provide detailed information regarding the major factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micromarkets concerning individual growth trends, prospects, and contributions to the Asia Pacific Pharmaceutical Filtration Market
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players in the Asia Pacific Pharmaceutical Filtration Market and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments such as product approvals & launches, expansions, agreements, and collaborations in the Asia Pacific Pharmaceutical Filtration Market
- To benchmark players within the Asia Pacific Pharmaceutical Filtration Market using the company evaluation matrix framework, which analyzes market players based on various parameters within the broad categories of business and service strategy
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific Pharmaceutical Filtration Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Asia Pacific Pharmaceutical Filtration Market