
Pharmaceutical Microfiltration Market Size, Growth, Share & Trends Analysis
Pharmaceutical Microfiltration Market by type (Membrane filter, Depth filter, Virus filter, Air Filter, Assemblies), Sterility Type (Sterile), Application (API, Protein), Scale, End User - Forecast to 2030




OVERVIEW
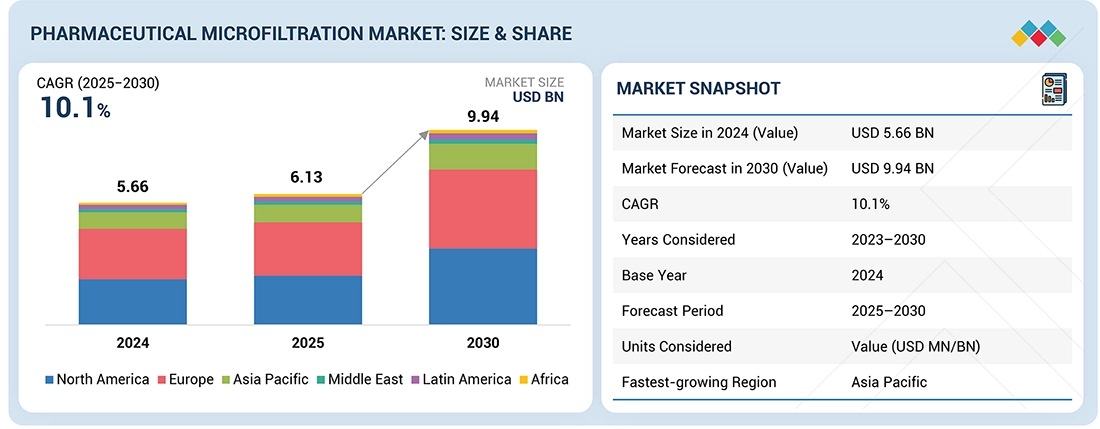
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
The global pharmaceutical microfiltration market, valued at US$5.66 billion in 2024, stood at US$6.13 billion in 2025 and is projected to advance at a resilient CAGR of 10.1% from 2025 to 2030, culminating in a forecasted valuation of US$9.94 billion by the end of the period. Pharmaceutical microfiltration is a membrane filtration process that utilizes membranes with defined pore sizes (typically ~0.1–1.0 µm) to remove cells, debris, and bioburden from pharmaceutical process fluids while allowing dissolved drugs and proteins to pass through.
KEY TAKEAWAYS
-
BY REGIONBy region, the Asia Pacific pharmaceutical microfiltration market is expected to be the fastest-growing regional segment, at a CAGR of 10.0% during the forecast period.
-
BY PRODUCTBy product, the consumables segment accounted for the largest share of 80% of the market in 2024.
-
BY STERILITY TYPEBy sterility type, sterile preparations are expected to dominate the pharmaceutical microfiltration market throughout the forecast period.
-
BY APPLICATIONBy application, the final product processing segment is expected to dominate the market.
-
BY END USERBy end user, the pharmaceutical & biopharmaceutical companies segment is expected to dominate the market.
-
COMPETITIVE LANDSCAPEMerck KGaA (Germany), Danaher Corporation (US), and Sartorius AG (Germany) were identified as the star players in the pharmaceutical microfiltration market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPEMeissner Filtration Products, Inc. (US), Amazon Filters Ltd. (UK), and Graver Technologies, LLC (US) have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging ma
The pharmaceutical microfiltration market is expanding rapidly, driven by the growing adoption of presterilized, gamma-stable microfilters and the shift toward single-use, closed processing systems in large-scale biologics and vaccine manufacturing.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The market for pharmaceutical microfiltration is undergoing significant changes, primarily due to evolving manufacturing processes, regulatory requirements, and therapeutic trends. A major driving force behind this shift is the increasing production of biologics, biosimilars, and advanced therapies, such as mRNA vaccines and cell & gene therapies. This surge in production is creating a higher demand for high-performance consumables, including depth filters, membrane filters, virus-retentive cartridges, and sterile single-use assemblies. As production lines scale up and transition towards multi-product, flexible facilities, manufacturers need ready-to-use, pre-validated filtration consumables that can ensure sterility, consistency, and operational efficiency across upstream, downstream, and final-fill workflows. The rise of single-use and hybrid bioprocessing platforms is a key factor in the growing demand for gamma-sterilized capsules, integrated fluid paths, and contamination-control consumables that comply with the requirements set by the FDA, EMA, and international GMP standards. CDMOs and biomanufacturers are increasingly relying on sterilizing-grade filters and high-capacity filter media. This reliance helps them reduce machine downtime while maintaining product quality and improving batch throughput.
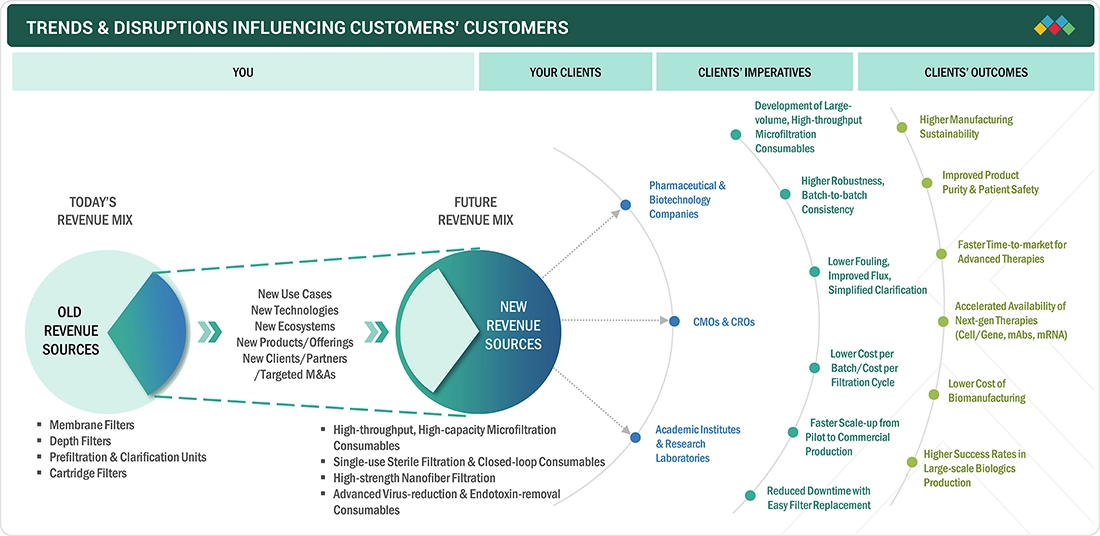
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Growing adoption of presterilized, gamma-stable microfilters

-
Expansion of microbial fermentation capacity
Level
-
Limitations of microfiltration in handling highly viscous or shear-sensitive biologics
Level
-
Growing demand for hybrid microfiltration modules
Level
-
Balancing filtration efficiency with protein recovery
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Growing adoption of presterilized, gamma-stable microfilters
The increasing use of presterilized, gamma-stable microfilters is driving market demand as pharmaceutical manufacturers prefer ready-to-use, contamination-free filtration solutions. These solutions simplify aseptic processing, reduce validation efforts, and support flexible production.
Restraint: Limitations of microfiltration in handling highly viscous or shear-sensitive biologics
The limitations of microfiltration in processing highly viscous or shear-sensitive biologics restrain market growth, as these products clog membranes quickly, reduce filtration efficiency, and risk product loss or structural damage during processing.
Opportunity: Growing demand for hybrid microfiltration modules
The demand for hybrid microfiltration modules is increasing as integrated micro-/ultrafiltration setups provide single-pass clarification, higher throughput, better product recovery, and more efficient workflows for modern biologics and advanced therapeutics manufacturing.
Opportunity: Balancing filtration efficiency with protein recovery
Balancing filtration efficiency with protein recovery is a major challenge, as tighter membranes improve particulate removal but can also retain or denature sensitive proteins, leading to reduced yields and inconsistent product quality.
PHARMACEUTICAL MICROFILTRATION MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Presterilized, gamma-stable microfilters and single-use assemblies for sterile filtration of biologics and vaccines in flexible, multi-product plants. | Simplifies sterilization, reduces contamination risk, and shortens turnaround times. |
 |
Gamma-stable capsule and cartridge microfilters integrated into single-use purification skids for clinical and commercial monoclonal antibody production. | Supports rapid batch changeovers, lowers cleaning validation burden, and improves flexibility. |
 |
Presterilized microfiltration capsules are used for the final sterile filtration of cell & gene therapy formulations in small-volume, high-value batches. | Minimizes product loss, enhances sterility assurance, and enables scalable, closed single-use processing. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The pharmaceutical microfiltration (MF) market is essentially supported by an intricate and interdependent ecosystem that includes membrane & media manufacturers, suppliers of single-use filtration components, system integrators, biopharmaceutical producers, CMOs/CDMOs, distributors, and end users. Manufacturers provide sterilizing-grade microfilters, hydrophilic polymer membranes, depth filters, preassembled single-use capsules, and high-throughput clarification modules that are specifically designed to meet stringent GMP and sterility standards. Biopharma companies and CDMOs use these microfiltration consumables that are necessary in upstream clarification, buffer/media preparation, downstream purification steps, and final aseptic processing to guarantee product safety and regulatory compliance. Engineering and integration partners facilitate the efficient implementation through their support in validation, automation-ready skid design, manifold configuration, and microfiltration workflow-targeted scale-up assistance. Distributors and supply chain partners ensure that there is no interruption in the availability of important microfilters and assemblies for production sites worldwide. This complex, interdependent ecosystem allows the contamination-controlled, high-efficiency manufacture of biologics, vaccines, and advanced therapeutics at the commercial scale.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
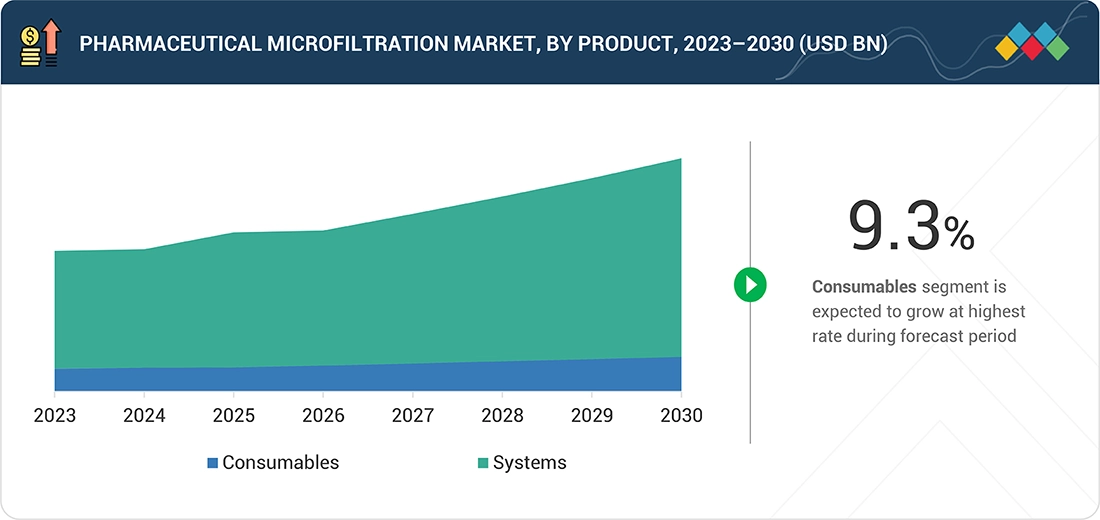
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Pharmaceutical Microfiltration Market, By Type
In?????? 2024, the consumables segment was the major contributor to the pharmaceutical microfiltration market, which was largely influenced by the growing demand for membrane cartridges, capsules, single-use assemblies, and depth media that are frequently consumed in large-batch biologics and vaccine runs.
Pharmaceutical Microfiltration Market, By Sterility Type
In 2024, sterile microfiltration consumables, such as validated sterilizing-grade membrane cartridges, single-use capsules, and syringe filters, accounted for the largest share of the pharmaceutical microfiltration market by sterility type. Their leading position is due to the increasing demand for microbial retention in biologics and parenteral formulations, where sterile microfiltration is used to guarantee patient safety and satisfy strict global regulatory ??????standards.
Pharmaceutical Microfiltration Market, By Application
In?????? 2024, the final product processing segment was the dominant application segment in the pharmaceutical microfiltration market. This is because microfiltration at this stage is very important in achieving the necessary product clarity and sterility before filling. The heavy reliance on strong, validated filters during commercial manufacturing makes these consumables necessary for batch release, product consistency, and GMP-compliant large-scale production.
Pharmaceutical Microfiltration Market, By End User
In 2024, pharmaceutical & biopharmaceutical companies dominated the pharmaceutical microfiltration market. These companies use microfiltration for various applications, such as upstream clarification, buffer/media preparation, intermediate purification steps, and final sterile filtration. The increasing pipelines of biologics, vaccines, and injectable drugs drive the continuous demand for high-performance microfilters and sterile-grade consumables/single-use assemblies that ensure product quality, regulatory compliance, and large-scale manufacturing output stability.
REGION
Asia Pacific to be fastest-growing region in market during forecast period
The Asia Pacific has emerged as one of the fastest-growing markets for pharmaceutical microfiltration. This growth is primarily driven by the rapid expansion of biologics and vaccine manufacturing in countries such as China, India, South Korea, and Singapore. The strong development pipelines for biosimilars and innovative biologics, combined with significant investments in regional CDMOs and large in-house biomanufacturing facilities, have led to a high demand for microfiltration consumables. These include sterilizing-grade cartridges, single-use capsules, depth filters, and virus-retentive membranes, which are essential for new large-scale production sites.
PHARMACEUTICAL MICROFILTRATION MARKET: COMPANY EVALUATION MATRIX
The pharmaceutical microfiltration market matrix identifies Merck KGaA (Star Player) as the leading company in the market. Merck offers a comprehensive range of high-efficiency filters suitable for both sterile and non-sterile applications, backed by a global presence and strong support in production and research environments. This leadership position has driven the adoption of Merck filters among biopharma companies, contract manufacturers, and hospital networks. Eaton Corporation (Emerging Leader) is distinguishing itself with innovative membrane technologies, adaptable product designs, and a growing local presence in precision therapeutic areas. Additionally, specialists focusing on niche filtration requirements are gaining market share due to their agile manufacturing practices and strategic initiatives, positioning them as potential future leaders in the industry.
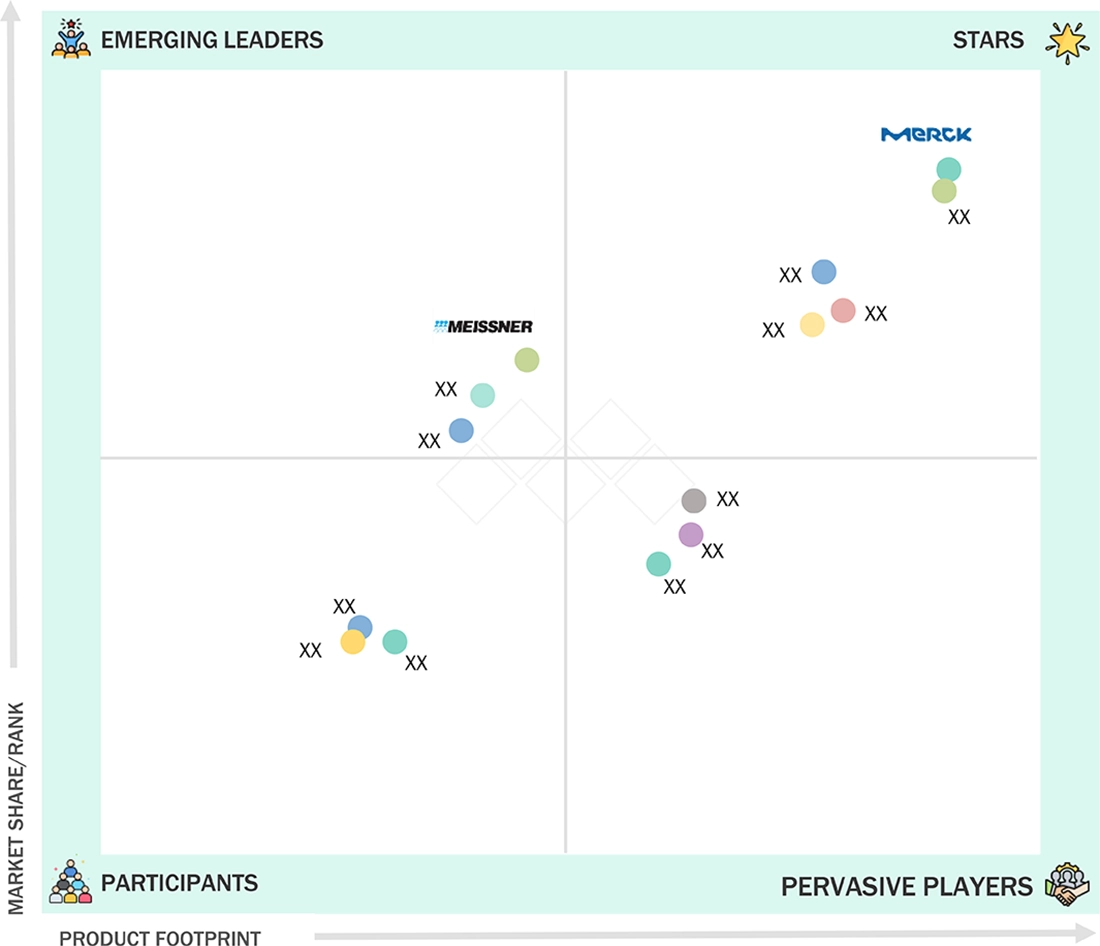
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Merck KGaA (Germany)
- Danaher Corporation (US)
- Sartorius AG (Germany)
- Parker-Hannifin Corporation (US)
- Eaton Corporation Plc (Ireland)
- Thermo Fisher Scientific Inc. (US)
- Solventum (US)
- Donaldson Company, Inc. (US)
- Porvair Plc (UK)
- Alfa Laval Corporation AB (Sweden)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 5.66 Billion |
| Market Forecast in 2030 (Value) | USD 9.94 Billion |
| Growth Rate | CAGR of 10.1% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
| Parent & Related Segment Reports |
Pharmaceutical Filtration Market North America Pharmaceutical Filtration Market Europe Pharmaceutical Filtration Market Asia Pacific Pharmaceutical Filtration Market Pharmaceutical Filtration Consumables Market |
WHAT IS IN IT FOR YOU: PHARMACEUTICAL MICROFILTRATION MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Usage insights by CDMOs/CMOs for microfiltration | Added step-wise detailing of microfiltration use: upstream clarification, harvest filtration, buffer/media preparation, downstream polishing, and sterile fill-finish operations. | Enables targeted positioning for high-volume, high-utilization stages of outsourced biomanufacturing, supporting strategic key-account focus and tailored value propositions. |
| Sustainability-focused microfiltration roadmap | Assessed footprint of single-use versus clean-in-place microfiltration trains, including plastic mass, energy for sterilization, and waste-handling requirements. | Supports ESG-driven customers with data-backed recommendations on low-waste microfiltration options and enables marketing of greener filtration solutions. |
RECENT DEVELOPMENTS
- October 2024 : Sartorius introduced the Sartoflow 1000 single-use TFF system, enabling efficient microfiltration and ultrafiltration for clinical-scale biologics with reduced cleaning and faster batch changeovers.
- June 2024 : Asahi Kasei launched a Microza hollow-fiber membrane system for water-for-injection production, offering an energy-efficient microfiltration alternative to traditional distillation in pharmaceutical plants.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the global Pharmaceutical Microfiltration Market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research:
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the Pharmaceutical Microfiltration Market. The secondary sources used for this study include American Membrane Technology Associations (AMTA), European Membrane Society (EMS), American Association of Pharmaceutical Scientists (AAPS), Pharmaceutical Research and Manufacturers of America (PhRMA), National Center for Biotechnology Information (NCBI), Parenteral Drug Association (PDA), Food and Drug Administration (FDA), European Medicines Agency (EMA), Health Canada, National Institutes of Health (NIH), World Health Organisation (WHO), Indian Pharmaceutical Association (IPA), International Society for Pharmaceutical Engineering (ISPE), BioProcess International Magazine, BioPharm International, Journal of Bioprocessing and Biotechniques, BioPlan Associates, ScienceDirect, research journals; corporate filings such as annual reports, SEC filings, investor presentations, and financial statements; press releases; trade, business, professional associations and among others. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research:
Extensive primary research was conducted after acquiring basic knowledge about the global Pharmaceutical Microfiltration Market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side, such as pharmaceutical and biopharmaceutical companies, CROs, CMOs, and academic & research institutes, and experts from the supply side, such as C-level and D-level executives, product managers, marketing & sales managers of key manufacturers, distributors, and channel partners. These interviews were conducted across six major regions, including the Asia Pacific, North America, Europe, Latin America, Middle East and Africa. Approximately 70% and 30% of the primary interviews were conducted with supply-side and demand-side participants, respectively. This primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
Market Size Estimation:
Both top-down and bottom-up approaches were used to estimate and validate the total size of the Pharmaceutical Microfiltration Market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation:
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition:
Membrane filtration is a process wherein solid particles present in a suspension are separated from a liquid or gas by employing membrane filters such as filter sheets, filter cartridges, or filter papers. These membrane filters are used to retain solids, thus allowing liquids to pass through. Membrane filters are used in pharmaceutical and biopharmaceutical manufacturing processes.
Stakeholders:
- Filter Manufacturers, Vendors, and Distributors
- Academic and Government Research Institutes
- Pharmaceutical and Biotechnology Companies
- Life Science Companies
- Venture Capitalists and Investors
- Government Organizations
- Private Research Firms
- Contract Research Organizations (CROs)
- Contract Development and Manufacturing Organizations (CDMOs)
Report Objectives:
- To define, describe, and forecast the global Pharmaceutical Microfiltration Market based on the product, technique, type, application, scale of operation and region
- To provide detailed information regarding the major factors influencing the growth of the market (such as drivers, restraints, opportunities and challenges)
- To strategically analyze micro-markets with respect to individual growth trends, future prospects, and contributions to the overall Pharmaceutical Microfiltration Market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to six main regions, namely, North America, Europe, Asia Pacific, Latin America, Middle East and Africa
- To strategically profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments such as acquisitions, product launches, expansions, and R&D activities in the Pharmaceutical Microfiltration Market.
Available Customizations:
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Portfolio Assessment:
- Product Matrix, which gives a detailed comparison of the product portfolios of the top three companies.
Company Information:
- Detailed analysis and profiling of additional market players (up to three).
Geographical Analysis:
- A further breakdown of the Rest of Asia Pacific Pharmaceutical Microfiltration Market into countries
- A further breakdown of the Rest of European Pharmaceutical Microfiltration Market into countries
- A further breakdown of the Rest of Latin American Pharmaceutical Microfiltration Market into countries
- A further breakdown of the Rest of Middle East Pharmaceutical Microfiltration Market into countries
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Pharmaceutical Microfiltration Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Pharmaceutical Microfiltration Market