
Asia Pacific Prefilled Syringes Market Size, Growth, Share & Trends Analysis
Asia Pacific Prefilled Syringes Market by Type (Conventional, Safety), Material (Glass Prefilled Syringe, Plastic Syringe), Design (Single-chamber, Dual-chamber), Application (Diabetes, Cancer, Rheumatoid Arthritis, Other), & Country - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
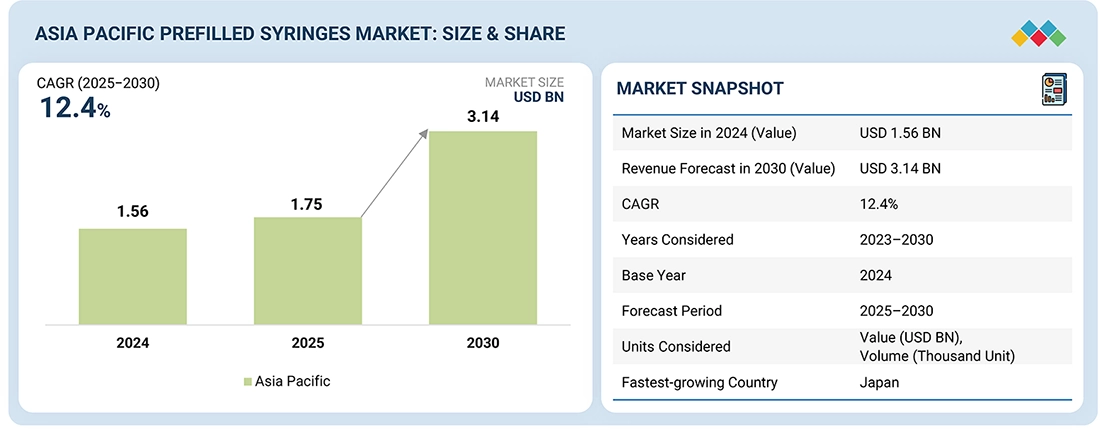
The Asia Pacific prefilled syringes market, valued at US$1.56 billion in 2024, stood at US$1.75 billion in 2025 and is projected to advance at a resilient CAGR of 12.4% from 2025 to 2030, culminating in a forecasted valuation of US$3.14 billion by the end of the period. Prefilled syringes are a convenient way to deliver parenteral medications or to use a single-dose packet with a premeasured dose. This drug administration method offers manufacturers advantages by reducing drug waste and extending product lifespan. Additionally, it provides ease of self-administration for patients at home and in non-hospital settings. Some examples of drugs packaged in prefilled syringes include biologics, vaccines, blood stimulants, therapeutic proteins, erythropoietin products, and interferons.
KEY TAKEAWAYS
-
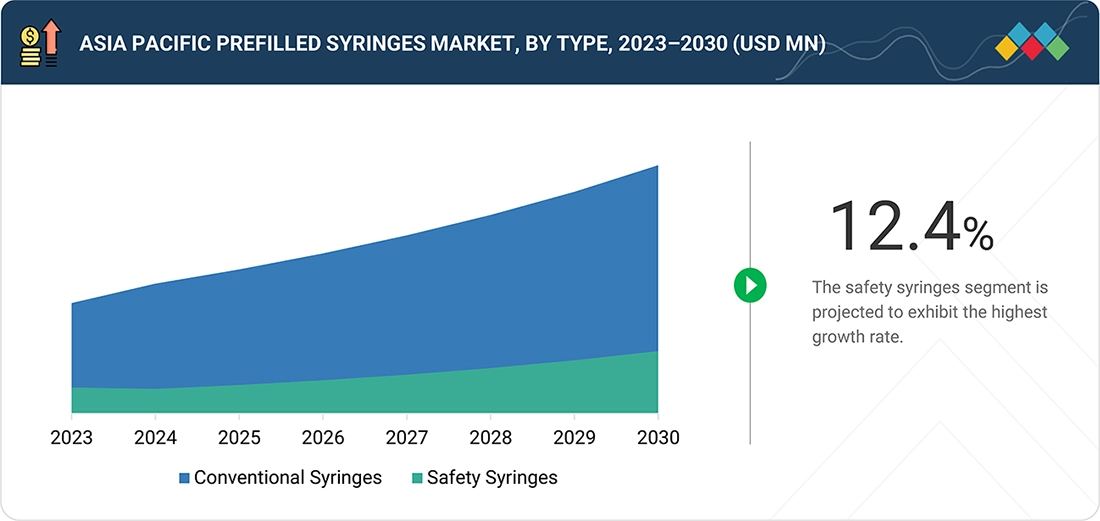
By TypeBy type, the conventional syringes segment dominated the Asia Pacific prefilled syringes market, accounting for 84.1% of the total share in 2024.
-
By DesignBy design, single-chamber prefilled syringes held the largest share of 63.9% of the Asia Pacific market in 2024.
-
By MaterialBy material, the glass segment led the Asia Pacific prefilled syringes market, accounting for 66.1% of the market share in 2024.
-
By ApplicationBy end user, the cancer segment is expected to grow at the highest CAGR in the Asia Pacific prefilled syringes market during the forecast period.
-
By CountryBy country, China is expected to grow at the highest CAGR during the forecast period.
-
COMPETITIVE LANDSCAPE- KEY PLAYERSBD, Gerresheimer AG, and Schott were identified as some of the star players in the Asia Pacific prefilled syringes market , given their strong market share and product/service footprint.
-
COMPETITIVE LANDSCAPE- STARTUPSCompanies such as Anthea Pharma and Medwise, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The Asia Pacific prefilled syringes market is propelled by the increasing prevalence of chronic diseases, the rising demand for self-administration, and the advantages of prefilled syringes, such as reduced risk of contamination and dosing errors. Additionally, technological advancements in drug delivery systems and the growing adoption of biologics are further accelerating market expansion. Supportive regulatory frameworks and increased focus on patient convenience and safety also contribute significantly to sustained growth.
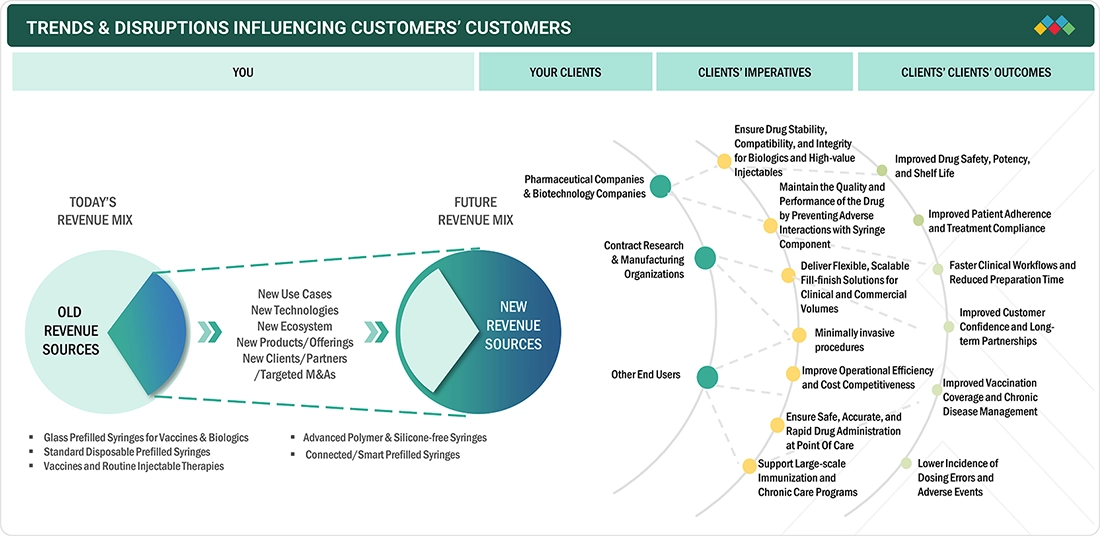
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The transition in the Asia Pacific prefilled syringes market is moving away from a traditional revenue model toward a future that emphasizes advanced technologies, such as polymer, silicone-free, and connected syringe solutions. This shift is driven by pharmaceutical companies, contract research organizations, manufacturing organizations, and healthcare end users, all of whom are demanding scalable, high-quality, and patient-centric injectable delivery options. These developments aim to enhance drug stability, improve patient safety, increase operational efficiency, and achieve better long-term treatment outcomes within the healthcare ecosystem.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing prevalence of chronic diseases

-
Growing adoption of self-injection devices
Level
-
Stringent government regulations
Level
-
Growing preference for unit-dose medication using prefilled syringes
-
Emerging markets with growing healthcare infrastructure
Level
-
Alternative drug delivery methods
-
Infections associated with needlestick injuries
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing prevalence of chronic diseases
The rising prevalence of chronic diseases is a key driver of the Asia Pacific prefilled syringes market. Conditions like diabetes, cardiovascular diseases, arthritis, and autoimmune disorders require consistent and often lifelong medication management. Prefilled syringes provide a convenient, safe, and efficient way to administer these medications, easing the burden on patients and healthcare systems. They ensure accurate dosing, reduce the risk of contamination and needlestick injuries, and enhance patient compliance by simplifying self-administration. Conditions like diabetes, cancer, neurological disorders, inflammatory bowel diseases, lung disorders, severe pancreatitis, and renal failure are major causes of death worldwide. As the world's population ages and chronic diseases become more common, the need for prefilled syringes will grow. These syringes are essential for patients who require regular, precise treatment. This growing demand is also driven by progress in biopharmaceuticals and the manufacturing of biologic drugs, which often require prefilled syringes due to their sensitivity to contamination and the need for accurate doses. Thus, as the prevalence of several chronic diseases worldwide increases, the demand for advanced, effective drug-delivery technologies is expected to rise, driving market growth for prefilled syringes.
Restraint: Stringent government regulations
Government regulations play a critical role in limiting the growth of the Asia Pacific prefilled syringes market by imposing strict requirements and lengthy approval processes for manufacturers. These regulations are in place to ensure the safety, efficacy, and quality of medical products, including both the drug and the delivery device. Prefilled syringes, classified as combination products, must meet strict criteria established by regulatory authorities. Prefilled syringes require thorough examination from both the pharmaceutical and medical device perspectives. Extensive clinical trials are necessary to demonstrate the safety and effectiveness of the combination, including stability studies, biocompatibility tests, and human trials. These trials are time-consuming and expensive, often requiring years of research and significant financial investment. The regulatory approval process demands detailed documentation on manufacturing processes, quality control measures, and post-market surveillance plans. Manufacturers must implement and maintain rigorous quality control systems that adhere to Good Manufacturing Practices (GMP) to ensure consistent product quality and safety. Meeting these requirements can delay product launches, as companies must thoroughly prepare and submit comprehensive data packages for review. While necessary for patient safety, the strict regulatory environment creates major obstacles to entering and expanding the market for prefilled syringes. The high costs and lengthy timelines required to comply with these regulations can discourage investment and innovation, thereby restraining market growth.
Opportunity: Emerging markets with growing healthcare infrastructure
The expanding healthcare infrastructure in emerging markets presents a promising opportunity for the Asia Pacific prefilled syringes market. These regions, which include many developing countries, are experiencing rapid growth in healthcare facilities, access to medical services, and overall healthcare spending. Prefilled syringes offer several advantages that align well with the needs of these emerging markets. Firstly, they provide enhanced safety and reliability in medication delivery. By prefilling precise doses of medications, prefilled syringes reduce the risk of dosing errors and contamination, which is crucial in settings where healthcare resources may be limited or where strict infection control measures are necessary. Secondly, the use of prefilled syringes can enhance healthcare efficiency by simplifying medication administration. They require less preparation time than traditional vials or ampoules. This allows healthcare providers to dedicate more time to patient care, ultimately reducing overall treatment times. This efficiency is particularly beneficial in environments with high patient volumes and limited healthcare staff. Additionally, prefilled syringes offer versatility and adaptability to meet diverse healthcare needs. They can be designed to accommodate various drug formulations and dosages, catering to the specific treatment requirements of local populations. This flexibility supports the delivery of a wide range of medications, including vaccines, biologics, and therapeutic agents, thereby addressing a broad spectrum of healthcare challenges prevalent in emerging markets.
Challenge: Alternative drug delivery methods
Alternative drug delivery methods pose a significant challenge to the Asia Pacific prefilled syringes market, affecting adoption rates and market growth. Several alternative delivery systems, each with unique advantages and applications, directly compete with prefilled syringes in various healthcare settings. Autoinjectors combine the convenience of prefilled syringes with automated injection mechanisms, making them popular for self-administration of medications. They are widely used for emergency treatments (e.g., epinephrine for allergic reactions) and chronic conditions (e.g., autoimmune diseases). Autoinjectors offer precise dosing, ease of use, and portability, which appeals to patients who prefer a user-friendly, ready-to-administer option. Pen injectors have become a strong competitor to prefilled syringes, especially for self-administration of medications. They offer similar convenience to prefilled syringes with a pre-measured dose and a user-friendly injection mechanism. This makes them ideal for patients requiring frequent injections for chronic conditions like diabetes. However, pen injectors may not be compatible with all medications or offer the same level of flexibility as prefilled syringes. For example, some pen injectors are designed for single use with a pre-filled cartridge, limiting the ability to adjust the dosage.
ASIA PACIFIC PREFILLED SYRINGES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced glass and polymer prefilled syringes with safety features and integrated drug-delivery compatibility for biologics and chronic therapies | Reduced medication errors, improved drug stability, enhanced patient safety, consistent dose accuracy, and improved usability for self-administration |
 |
High-quality glass and plastic prefilled syringes and cartridges for vaccines, biologics, and high-value injectables, supported by fill-finish and CDMO capabilities | Improved drug container integrity, scalability from clinical to commercial volumes, reduced contamination risk, and reliable supply continuity |
 |
Premium pharmaceutical glass tubing and prefillable syringe systems optimized for sensitive biologics and vaccines, with advanced quality control and defect-reduction technologies | Enhanced drug stability and shelf life, minimized particulate risk, superior dimensional accuracy, reduced breakage, and improved regulatory confidence |
 |
Integrated prefilled syringe components, elastomeric closures, and containment systems designed to protect injectable drugs throughout fill-finish and administration | Preservation of drug potency, reduced extractable risk, improved container-closure integrity, higher fill-finish efficiency, and lower batch failure rates |
 |
Advanced drug delivery systems and injectable components supporting prefilled syringe formats, including user-centric and combination-product solutions | Improved patient adherence, enhanced ease of use, differentiated drug delivery performance, reduced administration errors, and stronger product lifecycle value |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Asia Pacific prefilled syringes market comprises a highly interconnected healthcare ecosystem, with manufacturers, distributors, regulators, and end users each playing a critical role in ensuring safe, efficient, and scalable drug delivery. Manufacturers such as BD, SCHOTT, Gerresheimer, and West Pharmaceutical Services drive innovation through the development of advanced glass and polymer prefilled syringes, elastomeric components, and integrated drug–device solutions. Their emphasis on drug stability, biocompatibility, silicone-free systems, and patient-centered designs meets the growing demand for vaccines, biologics, and self-administered injectable therapies while improving safety and minimizing medication errors. Distributors, including McKesson, Cardinal Health, and Henry Schein, enable broad market access by leveraging extensive logistics networks and digital ordering platforms. They act as vital intermediaries connecting manufacturers with pharmaceutical companies, hospitals, clinics, and pharmacies, ensuring reliable supply, cold-chain integrity, and timely availability across care settings. End users, such as pharmaceutical and biotechnology companies (e.g., Novartis, Sanofi, Pfizer) rely on prefilled syringes to improve dosing accuracy, streamline clinical workflows, and support large-scale immunization and chronic disease management programs. Finally, regulatory bodies, including India's Ministry of Health and Family Welfare, China’s NMPA, and Japan’s PMDA, oversee product approval, quality standards, and safety compliance. Their regulations ensure patient protection, product reliability, and global harmonization, while reimbursement frameworks and healthcare policies directly influence adoption rates and commercial viability of advanced prefilled syringe technologies.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Asia Pacific Prefilled Syringes Market, by Type
By type, the Asia Pacific prefilled syringes market is segmented into conventional prefilled syringes and safety prefilled syringes. In 2024, the conventional prefilled syringes segment accounted for the largest share of the Asia Pacific prefilled syringe market, driven by widespread adoption, cost-effectiveness, and proven reliability. These syringes, which are preloaded with medication, offer significant advantages, including reduced medication errors, improved dosing accuracy, and enhanced patient safety. Their convenience for both healthcare professionals and patients, combined with the growing prevalence of chronic diseases requiring regular injections, further drives their dominance in the market. Additionally, advancements in syringe materials and manufacturing technologies have improved the safety and usability of conventional prefilled syringes, solidifying their market leadership.
Asia Pacific Prefilled Syringes Market, by Material
By material, the Asia Pacific prefilled syringes market is segmented into glass prefilled syringes and plastic prefilled syringes. The glass prefilled syringes segment dominates the Asia Pacific prefilled syringes market due to their superior chemical compatibility, which minimizes interactions with a wide range of pharmaceutical drugs, ensuring drug stability and efficacy. Their excellent barrier properties prevent oxygen and moisture from entering, preserving the medication's integrity over time. Additionally, glass' robust, inert nature makes it highly resistant to breakage and contamination, offering enhanced safety and reliability. The well-established manufacturing processes for glass syringes also contribute to their widespread adoption, providing consistency and quality assurance in drug delivery systems.
Asia Pacific Prefilled Syringes Market, by Design
By design, the Asia Pacific prefilled syringes market is segmented into single-chamber, dual-chamber, and customized prefilled syringes. In 2024, the single-chamber prefilled syringes segment has emerged as the dominant force in the Asia Pacific prefilled syringes market due to their numerous advantages, including ease of use, enhanced patient safety, and reduction in medication errors. These syringes are preloaded with a precise dose of medication, minimizing the need for manual preparation and decreasing the risk of contamination. Their convenience and reliability make them highly preferred in various medical settings, particularly for administering vaccines, biologics, and emergency medications. Furthermore, the growing trend toward self-administration of drugs and the increasing demand for advanced drug delivery systems are driving the adoption of single-chamber prefilled syringes, solidifying their leading position in the market.
Asia Pacific Prefilled Syringes Market, by Application
By application, the Asia Pacific prefilled syringes market is segmented into diabetes, rheumatoid arthritis, anaphylaxis, cancer, thrombosis, ophthalmology, and other applications. In 2024, the cancer segment is projected to hold the largest share in the Asia Pacific prefilled syringes market due to the rising prevalence of cancer globally, which necessitates frequent and precise administration of injectable medications. Prefilled syringes offer numerous advantages, including reducing the risk of dosage errors, minimizing contamination, and enhancing patient safety, which are critical in oncology treatments. The increasing adoption of biologics and biosimilars for cancer therapy, which often require parenteral administration, further drives the demand for prefilled syringes. Additionally, the convenience and efficiency they provide for both healthcare professionals and patients make prefilled syringes a preferred choice in cancer treatment regimens, contributing to their dominant market position.
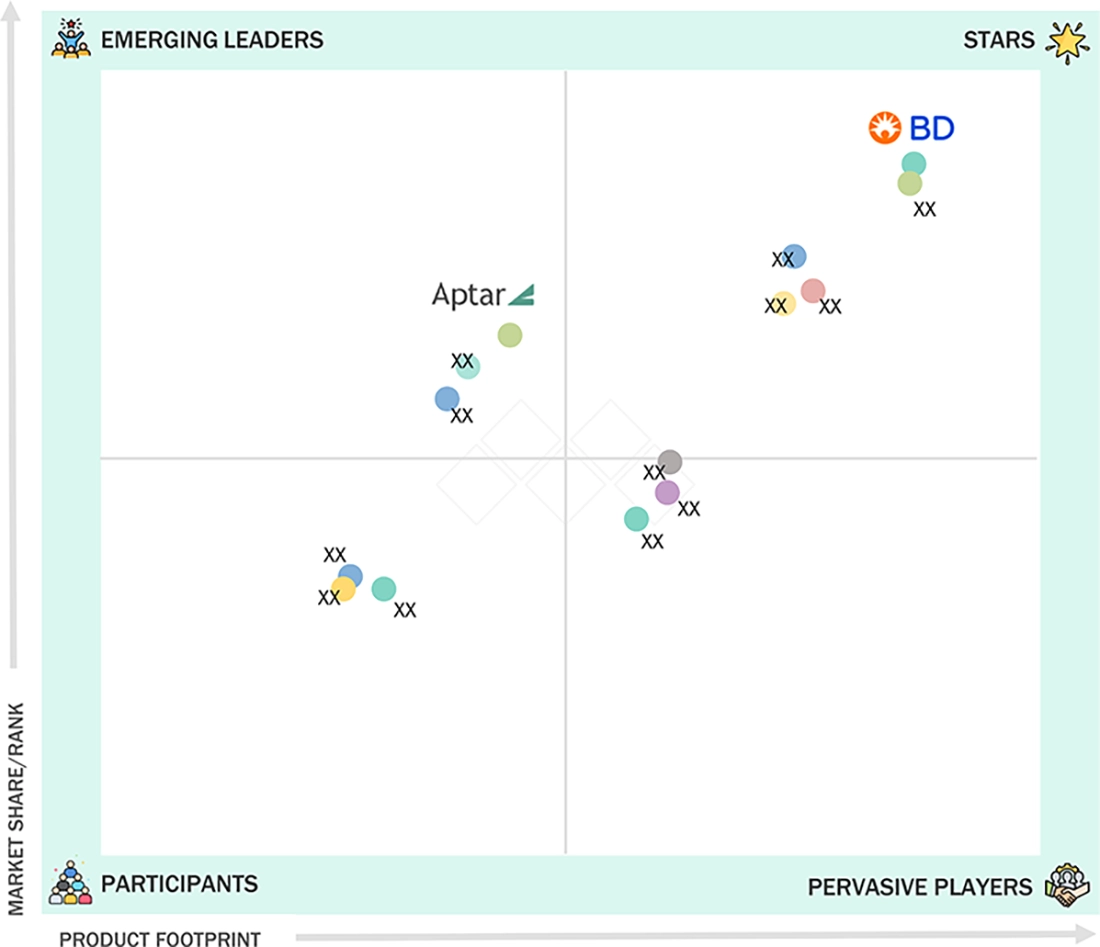
ASIA PACIFIC PREFILLED SYRINGES MARKET: COMPANY EVALUATION MATRIX
In the Asia Pacific prefilled syringes market matrix, BD (Star) leads with scale, extensive distribution, and a broad solutions portfolio. AptarGroup, Inc. (Emerging Leader) is gaining momentum with innovative products and packaging technologies. While BD dominates through reach, AptarGroup's innovation positions it for rapid growth toward the leaders quadrant.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- BD (US)
- Gerresheimer AG (Germany)
- Schott AG (Germany)
- Stevanto Group (Italy)
- West Pharmaceutical Services, Inc. (US)
- AptarGroup, Inc. (US)
- Nipro (Japan)
- Baxter (US)
- Owen Mumford Ltd. (UK)
- Weigao Medical International Co., Ltd. (China)
- Credence Medsystems, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 1.56 Billion |
| Revenue Forecast in 2030 (Value) | USD 3.14 Billion |
| Growth Rate | CAGR of 12.4% from 2025-2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million), Volume (Thousands Unit) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
WHAT IS IN IT FOR YOU: ASIA PACIFIC PREFILLED SYRINGES MARKET REPORT CONTENT GUIDE
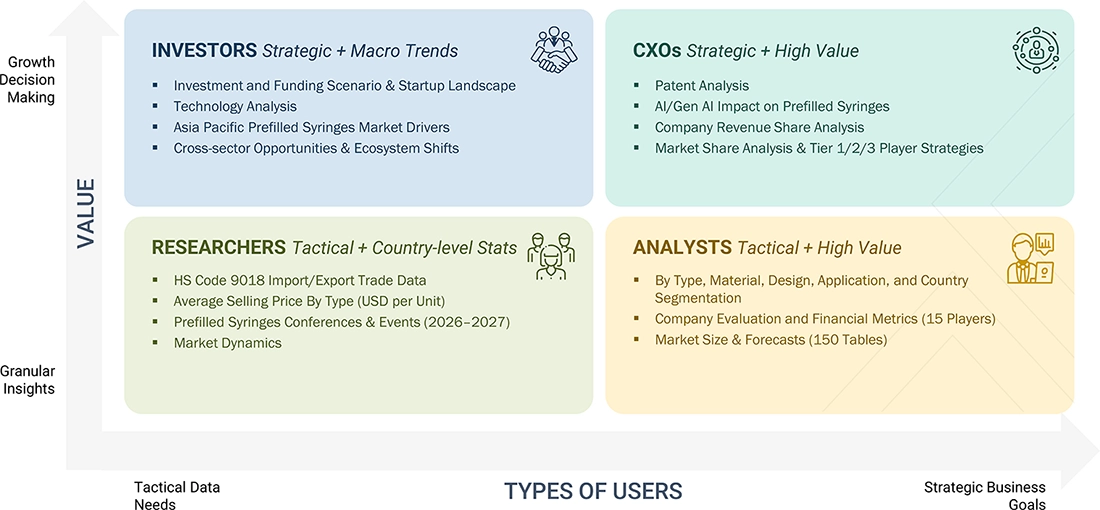
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis |
|
Identify interconnections and potential supply chain blind spots within injectable drug delivery ecosystem |
| Company Information | Key players: BD (US), Gerresheimer AG (Germany), SCHOTT (Germany),West Pharmaceutical Services, Inc. (US), and AptarGroup Inc. (US) | Insights on revenue shifts towards emerging innovations |
RECENT DEVELOPMENTS
- July 2024 : West Pharmaceutical Services, Inc. has expanded its presence in South Korea with a new office in Seoul and an upgraded warehouse in Hwaseong. This expansion enhanced West Pharmaceutical Services' ability to support injectable drug packaging and delivery, aligning with the country’s growing biologics sector.
- March 2023 : SCHOTT has invested USD 79 million (approximately INR 660 crore) over the past three years in expanding its pharmaceutical glass production in India in response to rising demand in Asia.
Table of Contents

Methodology
This study involved four major activities in estimating the current asia pacific prefilled syringes market size. Exhaustive secondary research was done to collect information on the market, peer market and parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the asia pacific prefilled syringes market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations operating in the asia pacific prefilled syringes market. The primary sources from the demand side included industry experts, purchase & sales managers, doctors, and personnel from research organizations. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
The research methodology used to estimate the size of the market includes the following details.
The market sizing of the market was undertaken from the global side.
Country-level Analysis: The size of the asia pacific prefilled syringes market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products and services in the overall asia pacific prefilled syringes market was obtained from secondary data and validated by primary participants to arrive at the total asia pacific prefilled syringes market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by asia pacific prefilled syringes and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall asia pacific prefilled syringes market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes explained above—the market was split into several segments and sub-segments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Asia pacific prefilled syringes are a convenient way to deliver parenteral medications or a single-dose packet of medication with a premeasured dose. This method of drug administration offers advantages for manufacturers in terms of reducing drug waste and extending product lifespan. Additionally, it provides ease of self-administration for patients at home and in non-hospital settings. Some examples of drugs packaged in asia pacific prefilled syringes include biologics, vaccines, blood stimulants, therapeutic proteins, erythropoietin products, and interferons.
Key Stakeholders
- Product manufacturers, distributors, and suppliers
- Research laboratories
- Academic universities and medical research centers
- R&D centers
- Business research and consulting firms
- Biotechnology companies
- Hospitals
- Clinical centers
- Pharmaceutical companies
- Medical research laboratories
- Consulting firms
Objectives of the Study
- To describe, analyze, and forecast the asia pacific prefilled syringes market by type, design, material, application, and region.
- To describe and forecast the asia pacific prefilled syringes market for key regions—North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and GCC Countries.
- To provide detailed information regarding the drivers, restraints, opportunities, and challenges influencing the growth of the asia pacific prefilled syringes market.
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall market.
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for market players.
- To profile key players and comprehensively analyze their market shares and core competencies2 in the asia pacific prefilled syringes market.
- To analyze competitive developments such as partnerships, collaborations, agreements & acquisitions, product launches, expansions, and R&D activities in the asia pacific prefilled syringes market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
Geographic Analysis
- Further breakdown of the Rest of Europe asia pacific prefilled syringes market into Belgium, Russia, the Netherlands, Switzerland, and other countries.
- Further breakdown of the Rest of Asia Pacific asia pacific prefilled syringes market into Indonesia, Philippines, Vietnam, Hong Kong, and other countries
- Further breakdown of the Rest of Latin America asia pacific prefilled syringes market into Colombia, Peru, and other countries.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Asia Pacific Prefilled Syringes Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in Asia Pacific Prefilled Syringes Market