
North America Prefilled Syringes Market Size, Growth, Share & Trends Analysis
North America Prefilled Syringes Market by Type (Conventional, Safety), Material (Glass Prefilled, Plastic), Design (Single-chamber, Dual-chamber), Application (Diabetes, Cancer, Rheumatoid Arthritis, Other), and Country - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
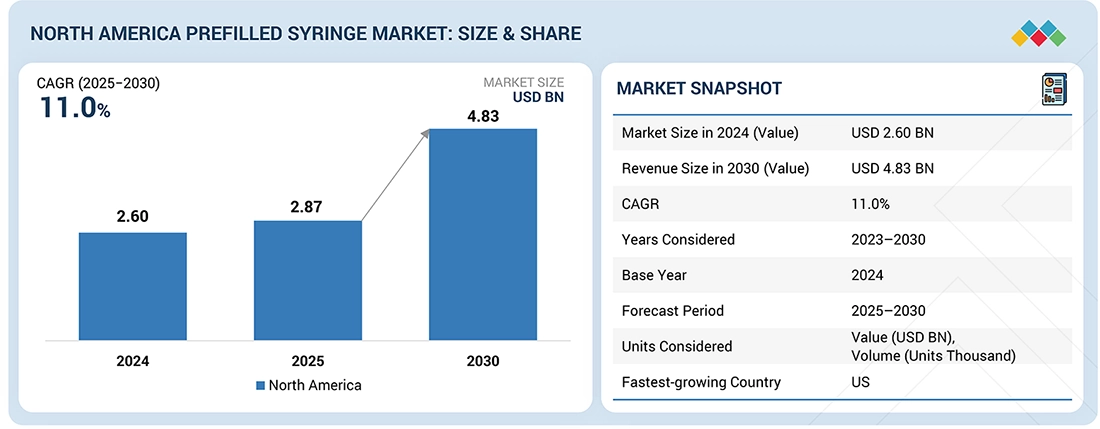
The North America prefilled syringes market, valued at US$2.60 billion in 2024, stood at US$2.87 billion in 2025 and is projected to advance at a resilient CAGR of 11.0% from 2025 to 2030, culminating in a forecasted valuation of US$4.83 billion by the end of the period. Prefilled syringes are a convenient way to deliver parenteral medications or to use a single-dose packet with a premeasured dose. This drug administration method offers manufacturers advantages by reducing drug waste and extending product lifespan. Additionally, it provides ease of self-administration for patients at home and in non-hospital settings. Some examples of drugs packaged in prefilled syringes include biologics, vaccines, blood stimulants, therapeutic proteins, erythropoietin products, and interferons.
KEY TAKEAWAYS
-
By CountryBy country, the US is expected to grow at the highest CAGR of 11.1% during the forecast period.
-
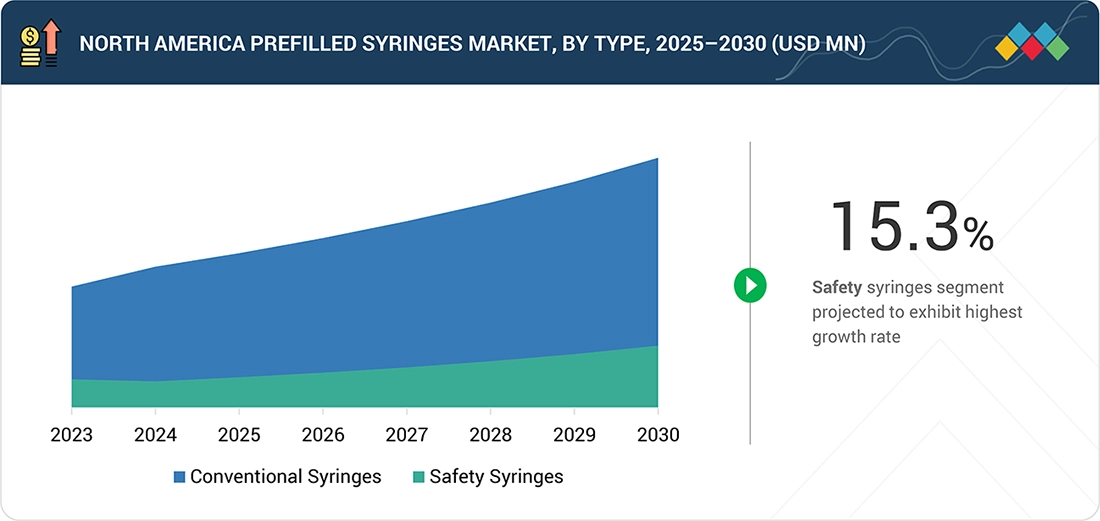
By TypeBy type, the North America prefilled syringes market was dominated by the conventional syringes segment, with a share of 84.1% in 2024.
-
By DesignBy design, single-chamber prefilled syringes held the leading position in the North America market, capturing 59.1% of the share in 2024.
-
By MaterialBy material, the glass segment dominated the North America prefilled syringes market, in 2024.
-
By ApplicationBy application, the cancer segment is expected to grow at the highest CAGR during the forecast period.
-
COMPETITIVE LANDSCAPE- KEY PLAYERSBD, Gerresheimer AG, and Schott were identified as some of the star players in the North America prefilled syringes market , given their strong market share and product/service footprint.
-
COMPETITIVE LANDSCAPE- STARTUPSCompanies such as 3CK and Sure MedTech, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The North America prefilled syringes market is propelled by the rising prevalence of chronic diseases, growing demand for self-administration, and the advantages of prefilled syringes, including reduced risk of contamination and dosing errors. Additionally, technological advancements in drug delivery systems and the growing adoption of biologics are accelerating market expansion. Supportive regulatory frameworks and increased focus on patient convenience and safety also contribute significantly to sustained growth.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
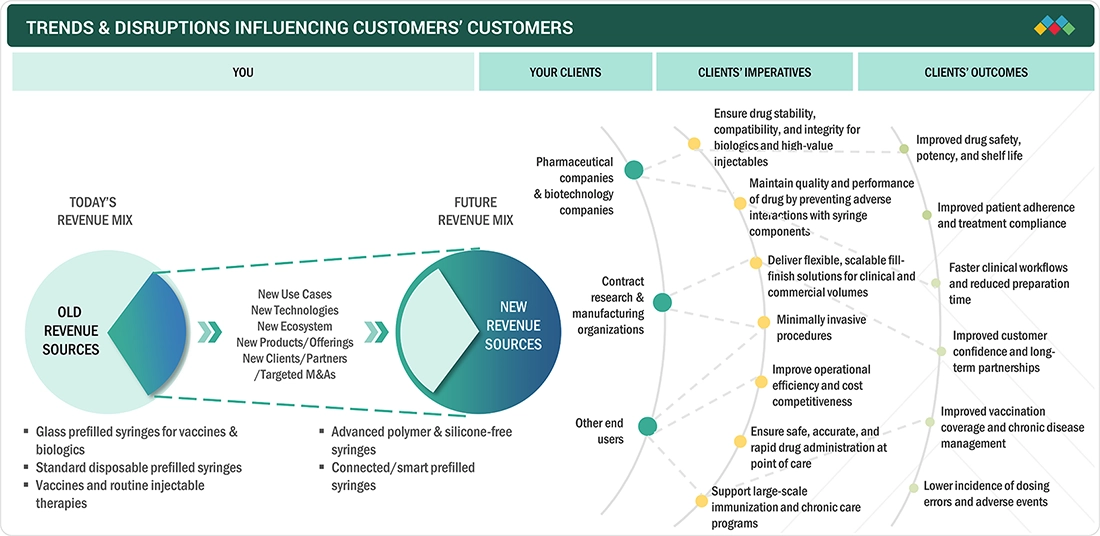
The North America prefilled syringes market is transitioning from a largely traditional revenue base toward a future dominated by advanced, polymer, silicone-free, and connected syringe technologies. It highlights how pharmaceutical companies, contract research and manufacturing organizations, and healthcare end users are driving demand for scalable, high-quality, and patient-centric injectable delivery solutions. These shifts aim to improve drug stability, patient safety, operational efficiency, and long-term treatment outcomes across the healthcare ecosystem.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Increasing prevalence of chronic diseases

-
Growing adoption of self-injection devices
Level
-
Stringent government regulations
Level
-
Growing preference for unit-dose medication using prefilled syringes
-
Strong healthcare infrastructure
Level
-
Alternative drug delivery methods
-
Infections associated with needlestick injuries
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Increasing prevalence of chronic diseases
The rising prevalence of chronic diseases, such as diabetes, cardiovascular issues, and autoimmune disorders, is driving growth in the North America prefilled syringes market. These syringes offer a convenient and efficient method for medication administration, ensuring accurate dosing and reducing the risk of contamination and needlestick injuries, thereby enhancing patient compliance. As conditions like cancer and neurological disorders become more common, there is an increasing need for prefilled syringes, especially given advancements in biopharmaceuticals that necessitate precise dosing due to contamination sensitivity. Consequently, demand for effective drug-delivery technologies is expected to rise, boosting the prefilled syringes market.
Restraint: Stringent government regulations
Government regulations play a critical role in limiting the growth of the prefilled syringes market by imposing strict requirements and lengthy approval processes on manufacturers. These regulations are designed to ensure the safety, efficacy, and quality of medical products, including both the drug and the delivery device. Prefilled syringes, classified as combination products, must meet strict criteria established by regulatory authorities. They require thorough evaluation from both pharmaceutical and medical device perspectives. Extensive clinical trials are necessary to demonstrate the safety and effectiveness of the combination, including stability studies, biocompatibility tests, and human trials. These trials are time-consuming and expensive, often requiring years of research and significant financial investment. The regulatory approval process demands detailed documentation of manufacturing processes, quality control measures, and post-market surveillance plans. Manufacturers must implement and maintain rigorous quality control systems, adhering to Good Manufacturing Practices (GMP), to ensure consistent product quality and safety. Meeting these requirements can delay product launches, as companies must prepare and submit comprehensive data packages for review. While necessary for patient safety, the strict regulatory environment creates major obstacles to entering and expanding the market for prefilled syringes. The high costs and lengthy timelines required to meet these regulations can discourage investment and innovation, thereby restraining the growth of the market..
Opportunity: Strong healthcare infrastructure
The advanced healthcare infrastructure of North America contributes significantly to a favorable environment for the use of prefilled syringes. The region has well-established hospital networks, specialty clinics, cold-chain logistics, and broad access to biologics and injectable therapies, which together make the transition to prefilled syringe formats faster and easier. Additionally, healthcare providers in the region prioritize safety, efficiency, and infection control, increasing the preference for ready-to-use, single-dose systems over conventional vial-and-syringe methods. This strong infrastructure supports large-scale immunization, chronic disease management, and specialty drug delivery, presenting continuous demand and opportunities for premium, safety-engineered prefilled syringe solutions.
Challenge: Alternative drug delivery methods
Alternative drug delivery methods pose a significant challenge to the North America prefilled syringes market, impacting adoption rates and market growth. Several alternative delivery systems, each with unique advantages and applications, directly compete with prefilled syringes in various healthcare settings. Autoinjectors combine the convenience of prefilled syringes with automated injection mechanisms, making them popular for self-administration of medications. They are widely used for emergency treatments (e.g., epinephrine for allergic reactions) and chronic conditions (e.g., autoimmune diseases). Autoinjectors offer precise dosing, ease of use, and portability, which appeals to patients who prefer a user-friendly, ready-to-administer option. Pen injectors have become a strong competitor to prefilled syringes, especially for self-administration of medications. They offer similar convenience to prefilled syringes with a pre-measured dose and a user-friendly injection mechanism. This makes them ideal for patients requiring frequent injections for chronic conditions like diabetes. However, pen injectors may not be compatible with all medications or offer the same level of flexibility as prefilled syringes. For instance, some pen injectors are designed for single use with a pre-filled cartridge, limiting the ability to adjust the dosage.
NORTH AMERICA PREFILLED SYRINGES MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Advanced glass and polymer prefilled syringes with safety features, and integrated drug-delivery compatibility for biologics and chronic therapies | Reduced medication errors, improved drug stability, enhanced patient safety, consistent dose accuracy, and improved usability for self-administration |
 |
High-quality glass and plastic prefilled syringes and cartridges for vaccines, biologics, and high-value injectables, supported by fill-finish and CDMO capabilities | Improved drug container integrity, scalability from clinical to commercial volumes, reduced contamination risk, and reliable supply continuity |
 |
Premium pharmaceutical glass tubing and prefillable syringe systems optimized for sensitive biologics and vaccines, with advanced quality control and defect-reduction technologies | Enhanced drug stability and shelf life, minimized particulate risk, superior dimensional accuracy, reduced breakage, and improved regulatory confidence |
 |
Integrated prefilled syringe components, elastomeric closures, and containment systems designed to protect injectable drugs throughout fill-finish and administration | Preservation of drug potency, reduced extractable risk, improved container-closure integrity, higher fill-finish efficiency, and lower batch failure rates |
 |
Advanced drug delivery systems and injectable components supporting prefilled syringe formats, including user-centric and combination-product solutions | Improved patient adherence, enhanced ease of use, differentiated drug delivery performance, reduced administration errors, and stronger product lifecycle value |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The North America prefilled syringes market is a highly interconnected healthcare ecosystem comprising manufacturers, distributors, regulators, and end users, each playing a critical role in ensuring safe, efficient, and scalable drug delivery. Manufacturers such as BD, SCHOTT, Gerresheimer, and West Pharmaceutical Services drive innovation through the development of advanced glass and polymer prefilled syringes, elastomeric components, and integrated drug–device solutions. Their focus on drug stability, biologic compatibility, silicone-free systems, and patient-centric designs supports the growing demand for vaccines, biologics, and self-administered injectable therapies while enhancing safety and reducing medication errors. Distributors, including McKesson, Cardinal Health, and Henry Schein, enable broad market access by leveraging extensive logistics networks and digital ordering platforms. They act as vital intermediaries connecting manufacturers with pharmaceutical companies, hospitals, clinics, and pharmacies, ensuring reliable supply, cold-chain integrity, and timely availability across care settings. End users, such as pharmaceutical and biotechnology companies (e.g., Novartis, Sanofi) rely on prefilled syringes to improve dosing accuracy, streamline clinical workflows, and support large-scale immunization and chronic disease management programs. Finally, regulatory bodies, including the US FDA, the US Centers for Disease Control and Prevention, and Health Canada, oversee product approval, quality standards, and safety compliance. Their regulations ensure patient protection, product reliability, and global harmonization—while reimbursement frameworks and healthcare policies directly influence adoption rates and commercial viability of advanced prefilled syringe technologies.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
North America Prefilled Syringes Market, By Type
By type, the North America prefilled syringes market is segmented into conventional and safety prefilled syringes. In 2024, the conventional prefilled syringe segment accounted for the largest share of the North America prefilled syringes market, driven by widespread adoption, cost-effectiveness, and proven reliability. These syringes, which come preloaded with medication, offer significant advantages, including reduced medication errors, improved dosing accuracy, and enhanced patient safety. Their convenience for both healthcare professionals and patients, combined with the growing prevalence of chronic diseases requiring regular injections, further drives their dominance in the market. Additionally, advancements in syringe materials and manufacturing technologies have improved the safety and usability of conventional prefilled syringes, solidifying their market leadership.
North America Prefilled Syringes Market, By Material
By material, the market is segmented into glass and plastic prefilled syringes. The glass prefilled syringe segment dominates the North America prefilled syringes market due to its superior chemical compatibility, which minimizes interactions with a wide range of pharmaceutical drugs, ensuring drug stability and efficacy. Its excellent barrier properties prevent oxygen and moisture from entering, preserving the medication's integrity over time. Additionally, the robust and inert nature of glass makes it highly resistant to breakage and contamination, offering enhanced safety and reliability. The well-established manufacturing processes for glass syringes also contribute to their widespread adoption, providing consistency and quality assurance in drug delivery systems.
North America Prefilled Syringes Market, By Design
By design, the North America prefilled syringes market is segmented into single-chamber, dual-chamber, and customized prefilled syringes. In 2024, the single-chamber prefilled syringe segment emerged as the dominant force in the North America prefilled syringes market due to its numerous advantages, including ease of use, enhanced patient safety, and a reduction in medication errors. These syringes are preloaded with a precise dose of medication, minimizing the need for manual preparation and decreasing the risk of contamination. Their convenience and reliability make them highly preferred in various medical settings, particularly for administering vaccines, biologics, and emergency medications. Furthermore, the growing trend toward self-administration of drugs and the increasing demand for advanced drug delivery systems contribute to the rising adoption of single-chamber prefilled syringes, solidifying their leading position in the market.
North America Prefilled Syringes Market, Application
By application, the North America prefilled syringes market is segmented into diabetes, rheumatoid arthritis, anaphylaxis, cancer, thrombosis, ophthalmology, and others. In 2024, the cancer segment is projected to hold the largest market share in the North America prefilled syringes market due to the rising global prevalence of cancer, which necessitates frequent and precise administration of injectable medications. Prefilled syringes offer numerous advantages, including reducing the risk of dosage errors, minimizing contamination, and enhancing patient safety, all of which are critical in oncology treatments. The increasing adoption of biologics and biosimilars for cancer therapy, which often require parenteral administration, further drives demand for prefilled syringes. Additionally, the convenience and efficiency they provide for both healthcare professionals and patients make prefilled syringes a preferred choice in cancer treatment regimens, contributing to their dominant market position.
NORTH AMERICA PREFILLED SYRINGES MARKET: COMPANY EVALUATION MATRIX
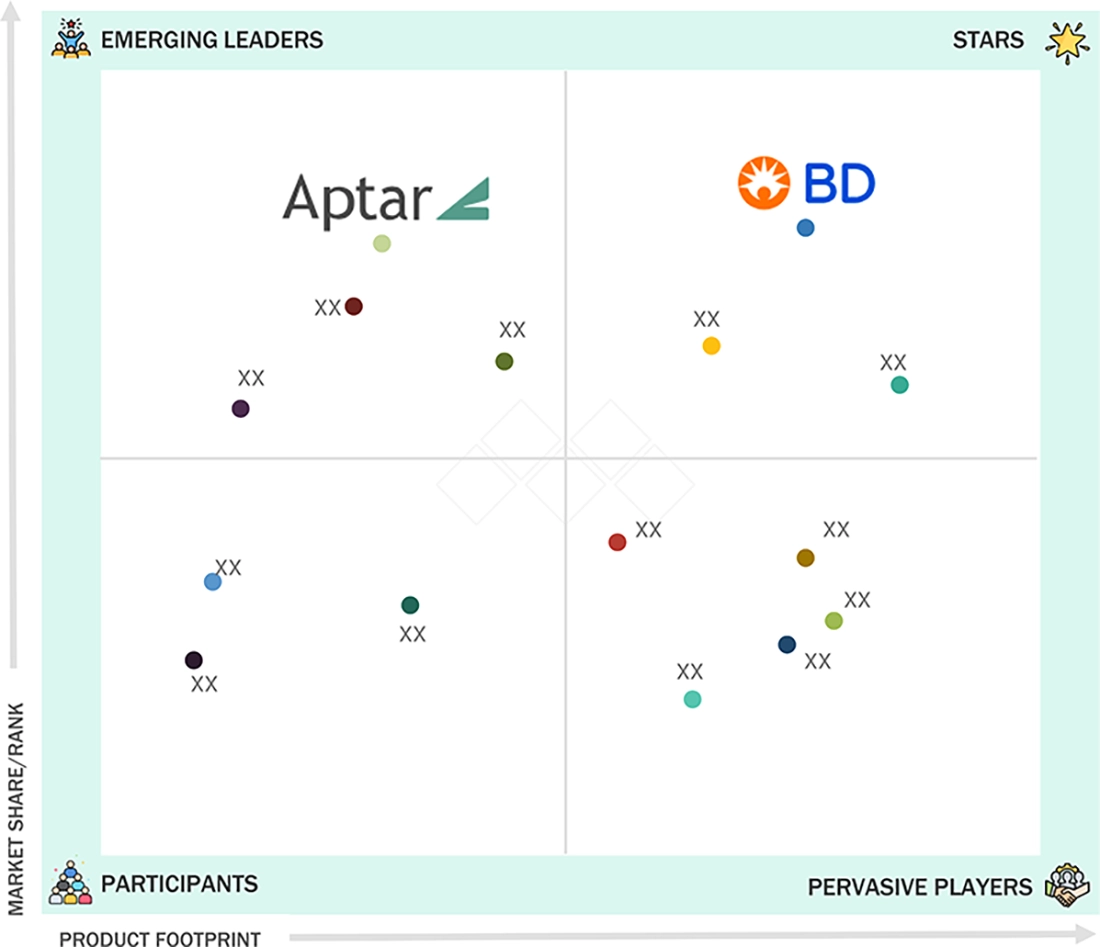
In the North America prefilled syringes market matrix, BD (Star) leads with scale, extensive distribution, and a broad solutions portfolio. APTARGROUP, INC. (Emerging Leader) is gaining momentum through innovative products and packaging technologies. While BD dominates through reach, APTARGROUP, INC.'s innovation positions it for rapid growth toward the leaders’ quadrant.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- BD (US)
- Gerresheimer AG (Germany)
- Schott AG (Germany)
- Stevanto Group
- West Pharmaceutical Services, Inc (US)
- AptarGroup, Inc (US)
- Nipro (Japan)
- Baxter (US)
- Owen Mumford ltd (UK)
- Weigao Medical International Co., Ltd (China)
- Credence Medsystems, Inc. (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.60 Billion |
| Revenue Size in 2030 (Value) | USD 4.83 Billion |
| Growth Rate | CAGR of 11.0% from 2025-2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million/Billion), Volume (Thousands Units) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
| Countries Covered | US, Canada |
WHAT IS IN IT FOR YOU: NORTH AMERICA PREFILLED SYRINGES MARKET REPORT CONTENT GUIDE
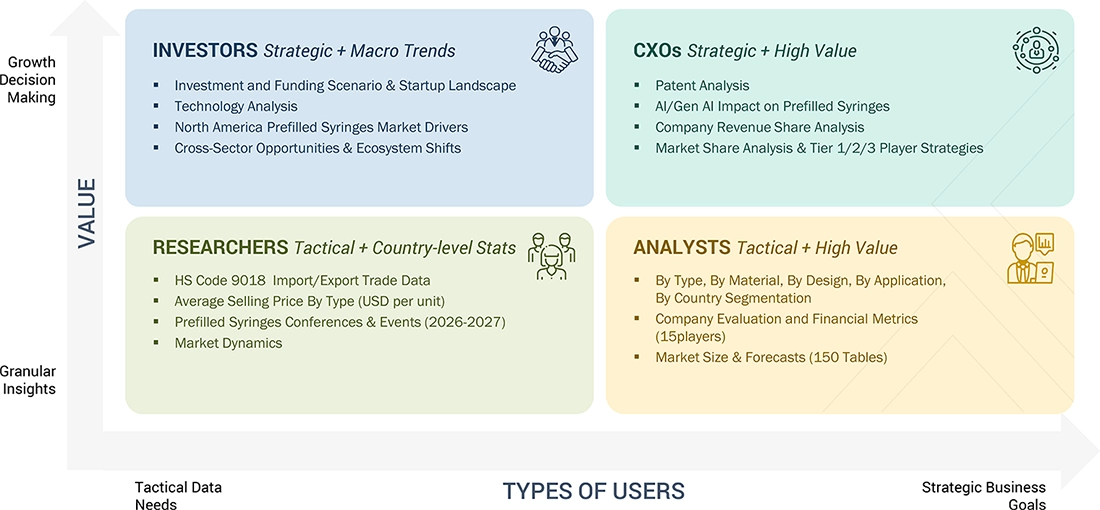
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Product Analysis |
|
Identify interconnections and potential supply chain blind spots within injectable drug delivery ecosystem |
| Company Information | Key players: BD (US), Gerresheimer AG (Germany) ,SCHOTT (Germany),West Pharmaceutical Services, Inc. (US), AptarGroup Inc. (US) | Insights on revenue shifts toward emerging innovations |
RECENT DEVELOPMENTS
- April 2025 : Gerresheimer partnered with Injecto to launch new silicone-oil- and PFAS-free syringe systems, expanding its portfolio with next-generation solutions designed to meet rising regulatory and drug-formulation requirements.
- October 2024 : BD collaborated with Ypsomed, to enhance self-injection solutions for high-viscosity biologic drugs. The collaboration integrated BD’s Neopak XtraFlow Glass Prefillable Syringe with Ypsomed’s YpsoMate 2.25 autoinjector, enabling efficient drug delivery for viscosities over 15cP. These initiatives improved patient experience and accelerated pharmaceutical product development.
Table of Contents

Methodology
This study involved four major activities in estimating the current north america prefilled syringes market size. Exhaustive secondary research was done to collect information on the market, peer market and parent market. The next step was to validate these findings, assumptions, and sizing with industry experts across the value chain through primary research. Both top-down and bottom-up approaches were employed to estimate the complete market size. After that, market breakdown and data triangulation were used to estimate the market size of segments and subsegments.
Secondary Research
The secondary research process involved the widespread use of secondary sources, directories, databases (such as Bloomberg Businessweek, Factiva, and D&B Hoovers), white papers, annual reports, company house documents, investor presentations, and SEC filings of companies. Secondary research was used to identify and collect information useful for the extensive, technical, market-oriented, and commercial study of the north america prefilled syringes market. It was also used to obtain important information about the key players and market classification and segmentation according to industry trends to the bottom-most level, and key developments related to market and technology perspectives. A database of the key industry leaders was also prepared using secondary research.
Primary Research
In the primary research process, various sources from both the supply and demand sides were interviewed to obtain qualitative and quantitative information for this report. The primary sources from the supply side include industry experts such as CEOs, vice presidents, marketing and sales directors, technology & innovation directors, and related key executives from various key companies and organizations operating in the north america prefilled syringes market. The primary sources from the demand side included industry experts, purchase & sales managers, doctors, and personnel from research organizations. Primary research was conducted to validate the market segmentation, identify key players in the market, and gather insights on key industry trends and key market dynamics.
To know about the assumptions considered for the study, download the pdf brochure
The research methodology used to estimate the size of the market includes the following details.
The market sizing of the market was undertaken from the global side.
Country-level Analysis: The size of the north america prefilled syringes market was obtained from the annual presentations of leading players and secondary data available in the public domain. The share of products and services in the overall north america prefilled syringes market was obtained from secondary data and validated by primary participants to arrive at the total north america prefilled syringes market. Primary participants further validated the numbers.
Geographic market assessment (by region & country): The geographic assessment was done using the following approaches:
Approach 1: Geographic revenue contributions/splits of leading players in the market (wherever available) and respective growth trends
Approach 2: Geographic adoption trends for individual product segments by north america prefilled syringes and growth prospects for each of the segments (assumptions and indicative estimates validated from primary interviews)
At each point, the assumptions and approaches were validated through industry experts contacted during primary research. Considering the limitations of data available from secondary research, revenue estimates for individual companies (for the overall north america prefilled syringes market and geographic market assessment) were ascertained based on a detailed analysis of their respective product offerings, geographic reach/strength (direct or through distributors or suppliers), and the shares of the leading players in a particular region or country.
Data Triangulation
After arriving at the overall market size—using the market size estimation processes explained above—the market was split into several segments and sub-segments. To complete the overall market engineering process and arrive at the exact statistics of each market segment and subsegment, data triangulation, and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
North america prefilled syringes are a convenient way to deliver parenteral medications or a single-dose packet of medication with a premeasured dose. This method of drug administration offers advantages for manufacturers in terms of reducing drug waste and extending product lifespan. Additionally, it provides ease of self-administration for patients at home and in non-hospital settings. Some examples of drugs packaged in north america prefilled syringes include biologics, vaccines, blood stimulants, therapeutic proteins, erythropoietin products, and interferons.
Key Stakeholders
- Product manufacturers, distributors, and suppliers
- Research laboratories
- Academic universities and medical research centers
- R&D centers
- Business research and consulting firms
- Biotechnology companies
- Hospitals
- Clinical centers
- Pharmaceutical companies
- Medical research laboratories
- Consulting firms
Objectives of the Study
- To describe, analyze, and forecast the north america prefilled syringes market by type, design, material, application, and region.
- To describe and forecast the north america prefilled syringes market for key regions—North America, Europe, the Asia Pacific, Latin America, the Middle East & Africa, and GCC Countries.
- To provide detailed information regarding the drivers, restraints, opportunities, and challenges influencing the growth of the north america prefilled syringes market.
- To strategically analyze micromarkets1 with respect to individual growth trends, prospects, and contributions to the overall market.
- To analyze market opportunities for stakeholders and provide details of the competitive landscape for market players.
- To profile key players and comprehensively analyze their market shares and core competencies2 in the north america prefilled syringes market.
- To analyze competitive developments such as partnerships, collaborations, agreements & acquisitions, product launches, expansions, and R&D activities in the north america prefilled syringes market.
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
Geographic Analysis
- Further breakdown of the Rest of Europe north america prefilled syringes market into Belgium, Russia, the Netherlands, Switzerland, and other countries.
- Further breakdown of the Rest of Asia Pacific north america prefilled syringes market into Indonesia, Philippines, Vietnam, Hong Kong, and other countries
- Further breakdown of the Rest of Latin America north america prefilled syringes market into Colombia, Peru, and other countries.
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the North America Prefilled Syringes Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation













Growth opportunities and latent adjacency in North America Prefilled Syringes Market