
Europe Cell Culture Market Size, Growth, Share & Trends Analysis
Europe Cell Culture Market by Product [Consumable {Media, Sera, Reagent, Vessel (Roller Bottle, Flask, Cell Factory)}, Equipment {Bioreactor, Centrifuge, Filtration, Incubator, Freezer}], Application [mAb, Vaccine, CGT], End User - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
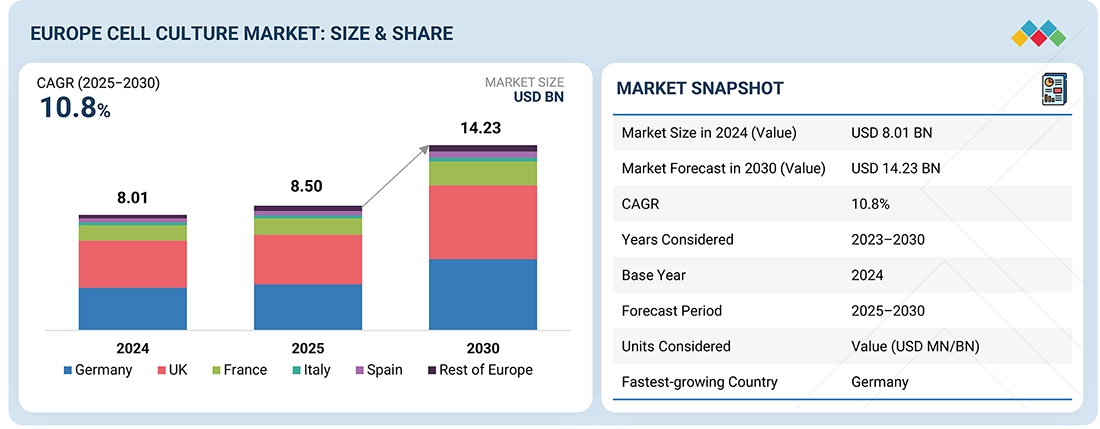
The Europe Cell Culture Market, valued at US$8.01 billion in 2024, stood at US$8.50 billion in 2025 and is projected to advance at a resilient CAGR of 10.8% from 2025 to 2030, culminating in a forecasted valuation of US$14.23 billion by the end of the period. The growth of the Europe cell culture market is driven by a strong and expanding biopharmaceutical sector, with rising production of biologics such as monoclonal antibodies, vaccines, and recombinant proteins that rely on sophisticated culture systems. The rising demand for advanced therapy medicinal products, including cell and gene therapies, combined with substantial public and private R&D funding and supportive EMA regulatory frameworks for regenerative and personalized medicine, is further accelerating the adoption of cell culture technologies across the region.
KEY TAKEAWAYS
-
By CountryThe German cell culture market accounted for a 27.6% revenue share in 2024.
-
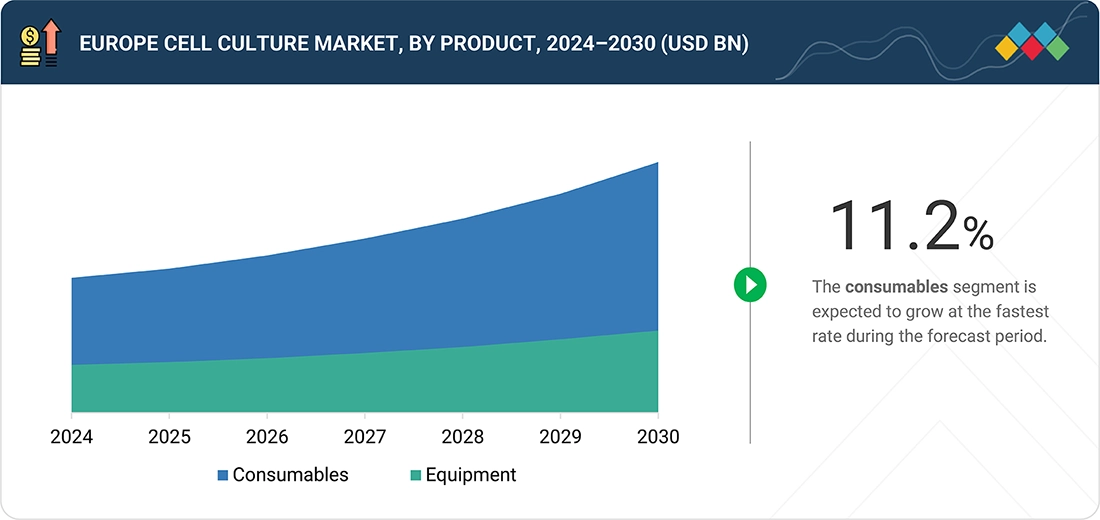
By ProductBy product, the consumables segment is expected to dominate the market in 2024.
-
By ApplicationBy application, the biopharmaceutical production segment is projected to grow at the fastest rate from 2025 to 2030.
-
By End userBy end user, the pharmaceutical & biotechnology companies segment is projected to grow at the fastest rate from 2025 to 2030.
-
Competitive LandscapeCompanies such as Thermo Fisher Scientific, Merck KGaA, and Sartorius AG were identified as some of the key players in the Europe cell culture market, given their strong market share and product footprint.
-
Competitive LandscapeCompanies such as Cellexus and Solida Biotech GmbH have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The cell culture market in Europe is rapidly expanding, driven by growing demand for advanced in vitro models and efficient bioprocessing units, which are key enablers of biologics manufacturing, precision medicine, and translational research. The increased use of cell culture in monoclonal antibody and vaccine production, as well as in cell and gene therapy activities, is accelerating market growth. Additionally, well-established regulatory routes from the EMA, steady R&D investment from both public and private sectors, and enthusiasm among biopharma companies, academic institutions, and research organizations for cell culture technology are the main factors contributing to the market growth.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
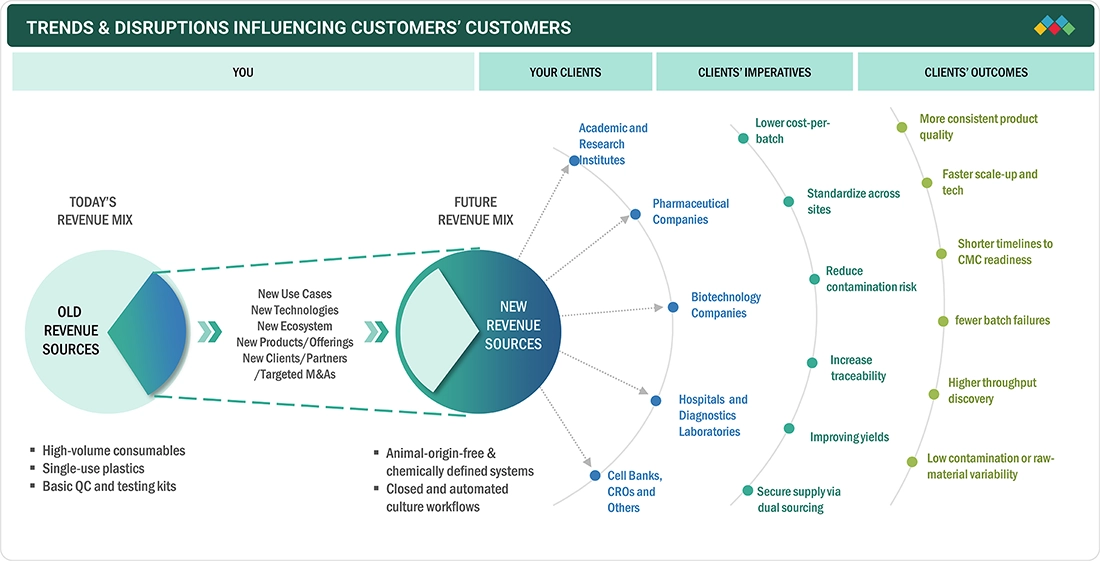
The impact on stakeholders' businesses in the Europe cell culture market is shaped by a shift toward biologics, advanced therapies, and more personalized care. The expansion of development and manufacturing programs by Biopharma companies and CDMOs keeps demand for media, reagents, single-use consumables, and scalable culture systems high. Academic and publicly funded research remains the backbone, continually providing new targets, cell models, and early-stage pipelines. Cell culture is central to the production of monoclonal antibodies, recombinant proteins, and vaccines; it is equally important for the development of cell and gene therapies. In addition, there is a constant transition to animal-origin-free and chemically defined inputs to align with quality standards and to streamline the regulatory process.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Biomanufacturing scale-up for vaccines and biologics

-
Strong publicly funded research base
Level
-
High cost of GMP transition and quality expectations
Level
-
Shift to animal-origin-free and chemically defined media
Level
-
Contamination risk and ongoing QC burden
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Biomanufacturing scale-up for vaccines and biologics
Across Europe, vaccine and biologics production is being scaled up, and many of these processes are cell-culture-based. More capacity means higher routine demand for media, supplements, buffers, and sterile single-use items. Scale-up also pushes teams to lock processes early and run more comparability work, which increases repeat testing and consumable pull-through. Vaccine manufacturing headlines, such as planned capacity ramp-ups in Europe, also reinforce long-term investments in cell-based production.
Restraint: High cost of GMP transition and quality expectations
Moving from research-grade work to GMP-grade production is costly in Europe, and the documentation burden is heavy. EU GMP Annex 1 raises expectations for contamination control, facility design, monitoring, and process discipline in sterile operations. Annex 1 is also stated to be fully applicable from 25 August 2024, keeping compliance activity high. For biologics, Annex 2 adds further expectations for controlling biological materials and ensuring process consistency. Together, these requirements drive validation, QC testing, and change-control work that add cost and time. Many buyers therefore delay vendor switches, limit new product trials, and stick to qualified materials longer.
Opportunity: Shift to animal-origin-free and chemically defined media
European users are steadily reducing animal-derived components because it simplifies risk management and improves consistency. EMA’s guideline on bovine serum underscores the need to control quality and safety when serum is used in manufacturing. This pushes more teams toward serum-free, animal-origin-free, and chemically defined media, where inputs are clearer and more controllable. Reviews also note that serum-free and chemically defined approaches can improve reproducibility and reduce contamination-related concerns. For suppliers, this creates room for premium formulations, tighter lot control, and stronger documentation packs. It also creates service opportunities in media optimization and process transfer for GMP programs.
Challenge: Contamination risk and ongoing QC burden
Contamination remains a constant threat in cell culture and creates recurring costs. ATCC notes that mycoplasma contamination in continuous cell cultures can range from 15–35%, underscoring the non-negotiable nature of routine testing in many labs. In regulated environments, Annex 1 expectations maintain pressure on monitoring, investigations, and prevention practices, adding to the workload. On top of that, the European Pharmacopoeia Commission has adopted revisions related to mycoplasmas, moving toward a less prescriptive, risk-assessment-based strategy. The direction is helpful, but it still demands strong validation and documentation choices. So, Europe’s cell culture teams continue to carry an ongoing QC burden alongside daily operations.
EUROPE CELL CULTURE MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Sartorius provided solutions (bioreactors, purification and fluid management technologies) for MabPlex’s large-scale mammalian cell culture facility for recombinant protein, mAb and ADC production | Reliable and easily scalable single-use technologies | Flexibility in production |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe cell culture market ecosystem comprises suppliers of media, sera, reagents, and consumables, led by global majors such as Thermo Fisher Scientific and Merck KGaA (Millipore Sigma), along with strong regional key players such as Sartorius. It also includes equipment providers supporting upstream and downstream workflows, such as bioreactors, incubators, biosafety cabinets, and single-use systems, where European manufacturing depth and engineering capability are clear differentiators. A vast network of specialized cell culture and cell bank providers supports research continuity and regulated manufacturing needs. End users include biopharmaceutical and biotechnology companies, academic and publicly funded research institutes, hospitals and clinical laboratories, and CROs/CMOs, reflecting Europe's balanced R&D-to-manufacturing footprint.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
North America cell culture market, By Product
As of 2024, consumables represent the largest share of the European cell culture market. This is mainly due to the fact that laboratories and manufacturers use disposable inputs on a daily basis. Media, supplements, serum replacements, buffers, and plastics are the major spend areas, as they are purchased continuously throughout routine cell maintenance, scale-up runs, and QC testing. The demand is supported by the biopharma and CDMO ecosystem in Europe, where stable production and process development keep the consumable pull-through at a high level.
North America cell culture market, By Application
In Europe, cell culture for biopharmaceutical production was the leading application in 2024 and is expected to retain the top position throughout the forecast period. This is because commercial and late-stage biologics manufacturing rely heavily on large-scale cell culture, continuously depleting media, feeds, and single-use systems. Upstream operations in Europe, grounded in the stable volumes of biologics manufacturers and CDMOs, will remain steady, driving recurring demand for cell culture inputs.
North America cell culture market, By End user
In Europe, pharmaceutical and biotechnology companies are the leading cell culture end users as of 2024. They make bulk purchases because cell culture is central to biologics and vaccine production, where media, feeds, and single-use consumables are used continuously. Europe's strong biopharma manufacturing footprint, supported by a mature CDMO network, is why development and commercial runs remain active across multiple hubs.
REGION
Germany to be the fastest-growing country in the Europe cell culture market during the forecast period
The German cell culture market is becoming the leading country in Europe to grow rapidly, due to the increase of scale-up activities in biologics, vaccines, and advanced therapies. The strong manufacturing capability and the large network of biopharma players and CDMOs are releasing more upstream capacity, resulting in a steady increase in demand for media, feeds, and high-turnover consumables. Moreover, Germany is endowed with a strong life-sciences R&D base, where translational programs rapidly transition from lab to pilot, resulting in a constant demand for reagents and culture systems.

EUROPE CELL CULTURE MARKET: COMPANY EVALUATION MATRIX
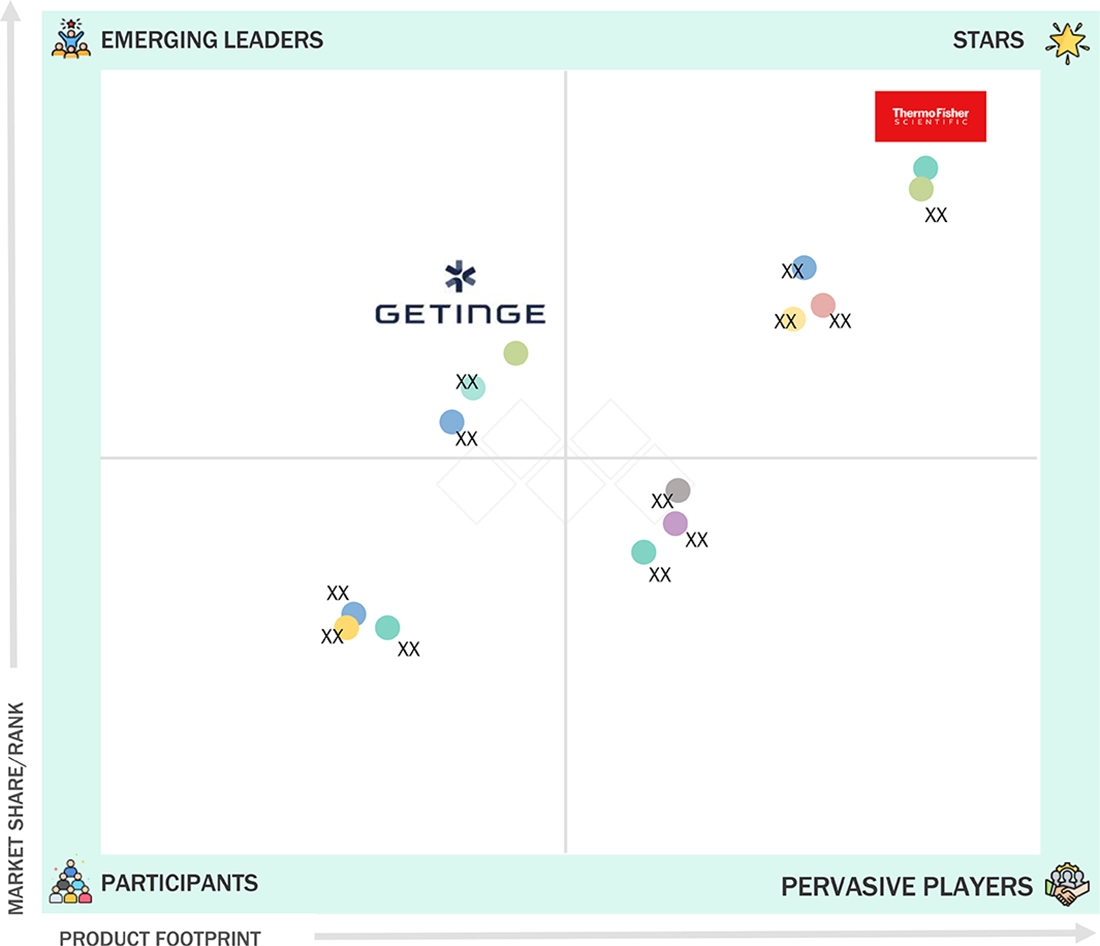
Thermo Fisher Scientific (Star) is the leader in the European cell culture market matrix, with a widespread presence in research labs, biopharma manufacturers, and CDMOs. The company's Gibco portfolio covers core cell culture media, supplements, sera/serum replacements, and routine reagents that are consumed regularly, thus strongly positioning Thermo Fisher in Europe's consumables-led market. Getinge (Emerging Leader) is consolidating its position in Europe by providing more efficient, compliant, and scalable cell culture operations through its bioprocessing and production support capabilities.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Thermo Fisher Scientific, Inc. (US)
- Danaher Corporation (US)
- Corning Incorporated (US)
- Merck KGaA (Germany)
- Sartorius AG (Germany)
- Eppendorf SE (Germany)
- Lonza (Switzerland)
- Getinge (Sweden)
- Miltenyi Biotec (Germany)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 8.50 Billion |
| Market Forecast in 2030 (Value) | USD 14.23 Billion |
| Growth Rate | CAGR of 10.8% from 2025-2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, UK, France, Italy, Spain, Rest of Europe |
| Parent & Related Segment Reports |
Cell Culture Market North America Cell Culture Market Asia Pacific Cell Culture Market Cell Culture Reagents Market Cell Culture Vessels Market |
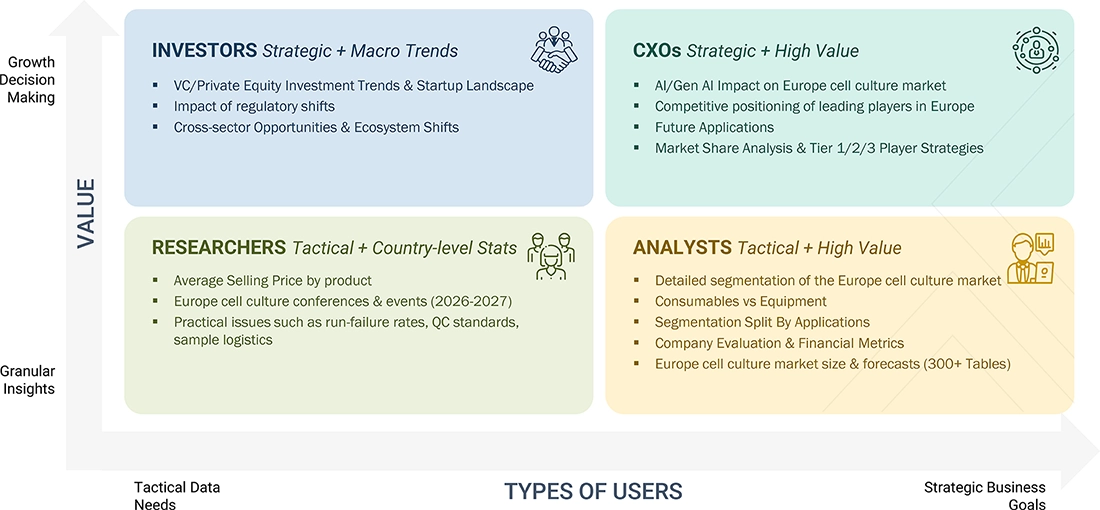
WHAT IS IN IT FOR YOU: EUROPE CELL CULTURE MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| German-based regenerative medicine company |
|
|
| UK-based biologics CDMO planning upstream expansion |
|
|
RECENT DEVELOPMENTS
- April 2025 : Sartorius AG (Germany) acquired MatTek (US), a leading developer and manufacturer of 3D microtissue models for ~USD 80 million, from BICO Group AB (Sweden). This acquisition will advance Sartorius’ cell culture portfolio.
- March 2025 : Bio-Techne (US) announced that it will open a new Customer Experience Centre in Düsseldorf, Germany (scheduled to open in summer 2026) to serve customers across the Europe, Middle East, and Africa (EMEA) region and support the company's regional growth strategy.
- September 2024 : Merck KGaA (Germany) launched the first single-use reactor specifically designed for the manufacture of antibody drug conjugates (ADCs).
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the Europe Cell Culture Market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The Europe market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the Europe Cell Culture Market. The secondary sources used for this study include World Health Organization (WHO), Food and Drug Administration, American Society for Cell Biology (ASCB), National Institutes of Health (NIH), International Society for Cell & Gene Therapy (ISCT), American Society for Gene and Cell Therapy (ASGCT), Centers of Disease Control and Prevention (CDC), International Serum Industry Association (ISIA), Pharmaceutical Research and Manufacturers of America (PhRMA), International Society for Vaccines (ISV), International Federation of Pharmaceutical Manufacturers & Associations (IFPMA), the Society for In Vitro Biology (SIVB), Japan Agency for Medical Research and Development, European Federation of Pharmaceutical Industries and Associations (EFPIA), American Type Culture Collection (ATCC), and the Alliance for Regenerative Medicine (ARM); corporate filings such as annual reports, SEC filings, investor presentations, and financial statements; press releases; trade, business, professional associations and among others. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
After acquiring foundational knowledge about the Europe Cell Culture Market through secondary research, extensive primary research was conducted. Multiple interviews were held with market experts from the demand side, including representatives from pharmaceutical and biopharmaceutical companies, hospitals & diagnostic laboratories, research & academic institutes, and CROs. Additionally, experts from the supply side were interviewed, including C-level and D-level executives, product managers, and marketing and sales managers from key manufacturers, distributors, and channel partners.
The primary data was collected through various methods, including questionnaires, emails, online surveys, personal interviews, and telephone interviews.
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the Europe Cell Culture Market. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable. The data was triangulated by studying various factors and trends from both the demand and supply sides.
Market Definition
Cell culture refers to removing cells from an animal or plant and their subsequent growth in an artificial environment under controlled conditions. It is a process of growing cells outside their natural environment, typically in a laboratory setting. This technique allows researchers to study cells in a controlled environment, providing insights into their behavior, growth, metabolism, and response to various stimuli.
Stakeholders
- Cell Culture Equipment and Reagents Manufacturers
- Analytical and Life Science Instrumentation Companies
- Academic and Research Institutes
- Pharmaceutical and Biotechnology Companies
- Life Science Companies
- Venture Capitalists and Investors
- Government Organizations
- Private Research Firms
- Contract Research Organizations (CROs)
- Hospitals and Diagnostic Laboratories
- Cell Banks
Report Objectives
- To define, describe, and forecast the Europe Cell Culture Market based on the product, application, end user, and region
- To provide detailed information regarding the major factors influencing the growth of the market (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micro-markets with respect to individual growth trends, future prospects, and contributions to the overall Europe Cell Culture Market
- To analyze opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To strategically profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments such as acquisitions, product launches, expansions, and R&D activities in the Europe Cell Culture Market
- To benchmark players within the Europe Cell Culture Market using the ‘Company Evaluation Matrix’ framework, which analyzes market players based on various parameters within the broad categories of business and product strategy
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Cell Culture Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Europe Cell Culture Market