
North America Nucleic Acid Isolation and Purification Market Size, Growth, Share & Trends Analysis
North America Nucleic Acid Isolation and Purification Market by Product (Kits, Reagents, Instruments), Method (Column, Magnetic Beads), Type (Genomic DNA, Plasmid DNA), Application (Diagnostics, Personalized Medicine), End User (CROs) - Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
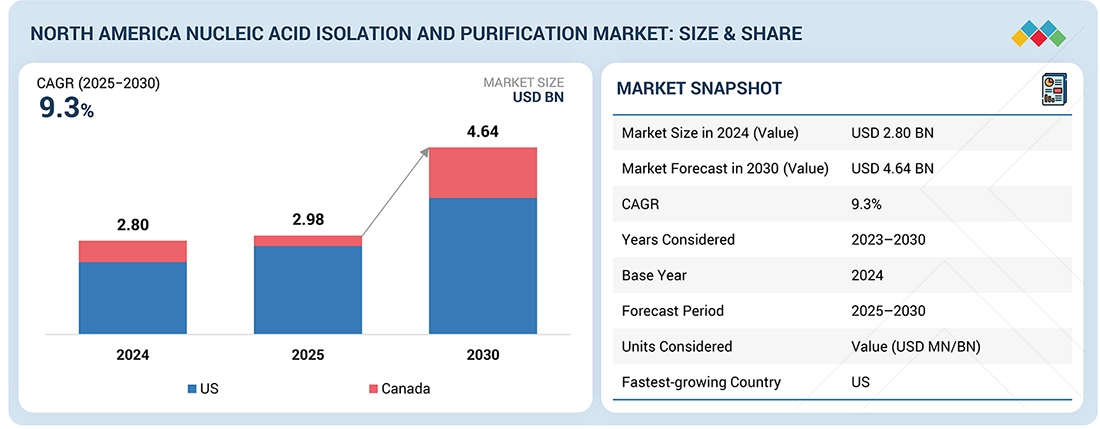
The North America Nucleic Acid Isolation and Purification Market, valued at USD 2.80 billion in 2024, stood at USD 2.98 billion in 2025 and is projected to advance at a resilient CAGR of 9.3% from 2025 to 2030, culminating in a forecasted valuation of USD 4.64 billion by the end of the period. The growth of the North America nucleic acid isolation and purification market is largely dependent on the region's substantial number of molecular tests and quick acceptance of NGS-based workflows. Clinical labs and reference labs require pure, inhibitor-free DNA/RNA for PCR, sequencing, and oncology panels. Additionally, demand is increasing in areas such as infectious disease testing, genetic screening, and liquid biopsy/circulating cell-free DNA (cfDNA) workflows. Labs are transitioning to automated, high-throughput extraction systems to save time and increase their reliability.
KEY TAKEAWAYS
-
By CountryThe US nucleic acid isolation and purification market accounted for a share of 87.4% in 2024.
-
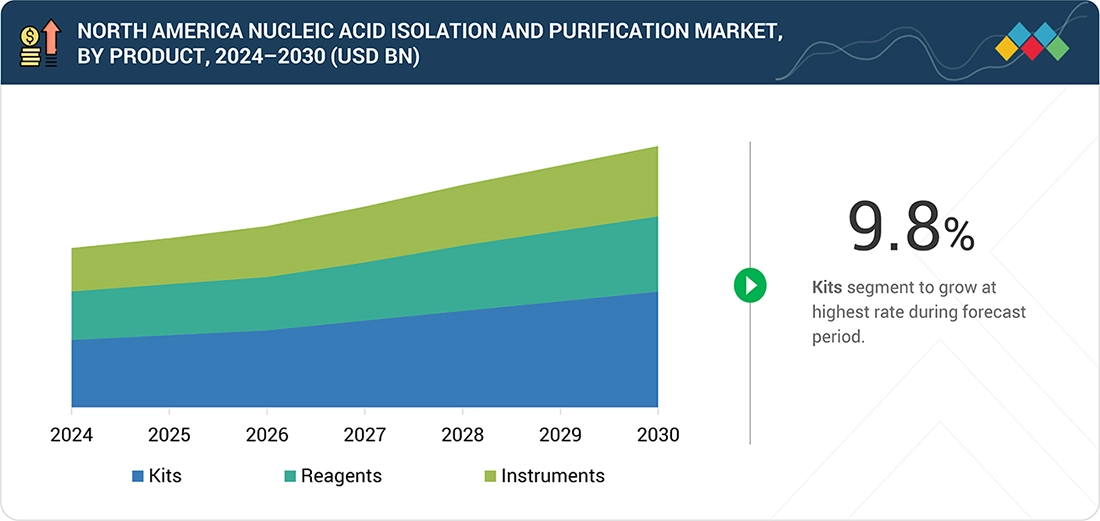
By ProductBy product, the kits segment is expected to register the highest growth rate of 9.8% during the forecast period.
-
By TypeBy type, the plasmid DNA isolation and purification segment dominated the market in 2024.
-
By MethodBy method, the magnetic bead-based isolation and purification segment dominated the market in 2024.
-
By ApplicationBy application, the personalized medicine segment is projected to grow at the fastest rate of 10.6% from 2025 to 2030.
-
By End UserBy end user, the pharmaceutical & biotechnology companies segment is projected to grow at the fastest rate from 2025 to 2030.
-
Competitive LandscapeCompanies such as Thermo Fisher Scientific, Danaher Corporation, and Merck KGaA are identified as key players in the North America nucleic acid isolation and purification market, due to their strong market share and extensive product footprint.
-
Competitive LandscapeCompanies like Omega Biotech, Inc. and Norgen Biotech Corp. have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The nucleic acid isolation and purification market in North America is growing with the rise in PCR and NGS-based testing in both clinical and research labs. There is an increasing need for pure and uniform DNA/RNA to cover oncology panels, infectious disease testing, genetic screening, and microbiome studies. Moreover, liquid biopsy and cfDNA workflows are introducing new sample types and setting more stringent performance requirements. In addition, laboratories are increasingly moving towards automated and high-throughput extraction platforms to reduce the time staff must spend hands-on and to increase reproducibility. The strong uptake in the network of reference labs, academic centers, and CROs/CDMOs is the main factor sustaining the momentum in the region.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The North America nucleic acid isolation and purification market is significantly influenced by the shift toward molecular decision-making in healthcare and life sciences. As techniques like PCR, qPCR, and NGS become routine in testing for infectious diseases, oncology profiling, and genetic screening, laboratories increasingly require reliable and high-quality DNA/RNA extraction as a vital first step. Key users include clinical laboratories, reference labs, and public health networks, which process large volumes of samples and need speed, consistency, and traceability. Additionally, biopharmaceutical and biotechnology companies contribute to the demand through bioanalytical studies, quality control testing, and analytics for cell and gene therapy, where the purity and removal of inhibitors are crucial for downstream success.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
High volume of PCR/NAAT testing across clinical labs

-
Expanding NGS workflows in research and biopharma testing
Level
-
High cost pressure
Level
-
Automation-first extraction to improve consistency and capacity
-
Growth in cfDNA, liquid biopsy, and low-input workflows
Level
-
Staffing shortages and pressure for faster throughput
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: High volume of PCR/NAAT testing across clinical labs
North America offers a wide range of nucleic acid-based tests, which require clean DNA/RNA before amplification. Laboratories also prioritize fast turnaround times and consistent yields. This ongoing demand drives the need for extraction columns, beads, and automated extraction systems. Even small improvements in yield can significantly reduce the need for repeat runs and minimize reporting delays. Therefore, extraction is a critical step in routine testing.
Restraint: High cost pressure
Extraction consumables significantly increase the cost per test. Spin columns tend to be more time-consuming and expensive than beads. High-volume labs are particularly affected by this. Due to budget constraints, laboratories often cut back on repeat tests and reduce waste. Some teams may also postpone upgrades, even when limited throughput is an issue. This delay can extend the replacement cycles for premium kits and systems.
Opportunity: Automation-first extraction to improve consistency and capacity
Automation in laboratories enables the efficient management of high volumes of work with minimal manual intervention. It enhances consistency and repeatability across different shifts and locations. Additionally, it supports barcoding and traceability requirements, making it particularly beneficial for large reference laboratories and hospital networks. Automation also effectively addresses staffing shortages, leading to an increasing demand for walk-away platforms and closed consumables.
Challenge: Staffing shortages and pressure for faster throughput
Many laboratories in North America are currently experiencing ongoing staffing challenges. The hands-on extraction process is both repetitive and time-sensitive, which makes short staffing a significant concern as it increases the risk of errors and delays in reporting results. As a result, labs are calling on vendors for more streamlined workflows and automation support. However, the implementation of automation still requires training and effective change management. Balancing speed, quality, and staffing remains a practical challenge for these laboratories.
NORTH AMERICA NUCLEIC ACID ISOLATION AND PURIFICATION MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
MacroGenics’ cell line development team was evaluating a new cell line and expression system. They needed a reliable platform, media, and feed setup that can scale without losing performance. They tested Gibco Efficient-Pro Medium and Feeds against their existing platform medium, then scaled the process from small studies into 500 L cGMP production. | Performance stayed consistent during scale-up, including at 500 L. The new platform process was also established within one year, helping to speed up execution and reduce rework. |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The North America nucleic acid isolation and purification market ecosystem includes suppliers of extraction kits and reagents (lysis buffers, silica columns, magnetic beads, wash/elution chemistries), along with automation platform providers offering manual-to-fully automated extraction systems. It also includes manufacturers of sample collection and stabilization products (such as swabs, transport media, blood tubes for cfDNA, and RNA preservatives), as well as plasticware and contamination-control consumables that support clean workflows. A growing base of clinical diagnostic labs, reference labs, public health labs, and academic research centers drives routine demand for DNA/RNA prep. On the other hand, biopharma and biotech companies use these solutions for bioanalytical testing, QC, and genomic workflows, while CROs/CDMOs scale extraction needs through multi-site studies and outsourced lab services.
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
North America Nucleic Acid Isolation and Purification Market, By Product
As of 2024, kits are the dominant choice in the North America nucleic acid isolation and purification market. This is primarily due to the reliance on consumables for extraction work. Laboratories conduct DNA and RNA preparations on a daily basis, frequently purchasing spin-column kits, magnetic bead kits, and buffer reagent kits. Kits are preferred because they are validated, standardized, and easy to use across different operators and locations. The demand for these kits is expected to remain strong across PCR and NGS workflows, including applications in infectious disease testing, oncology profiling, and genetic screening.
North America Nucleic Acid Isolation and Purification Market, By Type
In 2024, the plasmid DNA isolation and purification segment dominated the nucleic acid isolation and purification market in North America. This predominance is due to the fact that plasmid preparation is a routine, repeat-use process in research laboratories and biotech teams. Plasmids play a crucial role in cloning, vector construction, and the production of transfection-ready DNA. The demand for plasmid DNA is further strengthened by its use in cell and gene therapy as well as vaccine development, where it serves as a key starting material for viral vectors and related processes.
North America Nucleic Acid Isolation and Purification Market, By Method
As of 2024, the North America market for nucleic acid isolation and purification is primarily dominated by magnetic bead-based methods. This trend can be attributed to the region's growing emphasis on automation and high-throughput sample processing. Clinical laboratories and large reference labs favor bead workflows because they reduce hands-on time and provide consistent yields across different batches. Additionally, bead-based methods effectively process a wide variety of sample types, including blood, swabs, tissue, and saliva, demonstrating strong performance across these applications.
North America Nucleic Acid Isolation and Purification Market, By Application
As of 2024, the diagnostics application segment dominates the North America nucleic acid isolation and purification market. This is primarily due to the fact that DNA and RNA extraction is a crucial first step in many routine molecular tests. Hospitals, reference laboratories, and public health labs perform high volumes of PCR and NAAT assays, each of which starts with the isolation of nucleic acids. Additionally, there is a growing demand for molecular diagnostics in areas such as respiratory infections, sexually transmitted infection (STI) testing, transplant monitoring, and oncology profiling.
North America Nucleic Acid Isolation and Purification Market, By End User
In 2024, hospitals & diagnostic centers were the primary end users in the North America nucleic acid isolation and purification market. This dominance stems from the high volume of routine molecular testing conducted in these facilities, where DNA and RNA extraction is a crucial initial step. Hospital laboratories and diagnostic centers handle a variety of tests, including infectious disease panels, respiratory testing, STI screening, and oncology-related assays. Consistent sample preparation is essential to minimize the need for repeat tests and to ensure timely turnaround times.
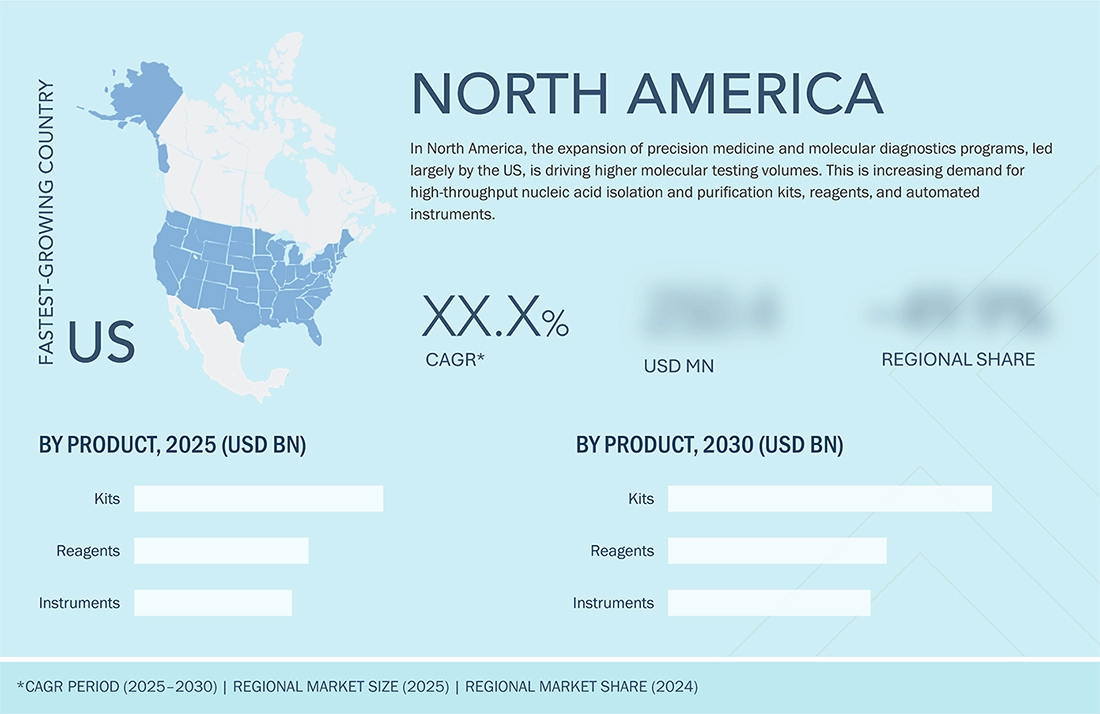
REGION
The US to be fastest-growing country in North America nucleic acid isolation and purification market during forecast period
The US nucleic acid isolation and purification market is emerging as the fastest-growing in North America, driven by its dominant revenue base, extensive genomic and molecular diagnostics infrastructure, and the rapid uptake of automated nucleic acid extraction platforms. Moreover, the rising penetration of molecular diagnostics and companion diagnostics across oncology, infectious disease, and hereditary disorders is a major factor behind the accelerated uptake of high-throughput nucleic acid isolation workflows, thereby increasing the need for specialized purification chemistries and seamlessly compatible automation platforms in the US market.
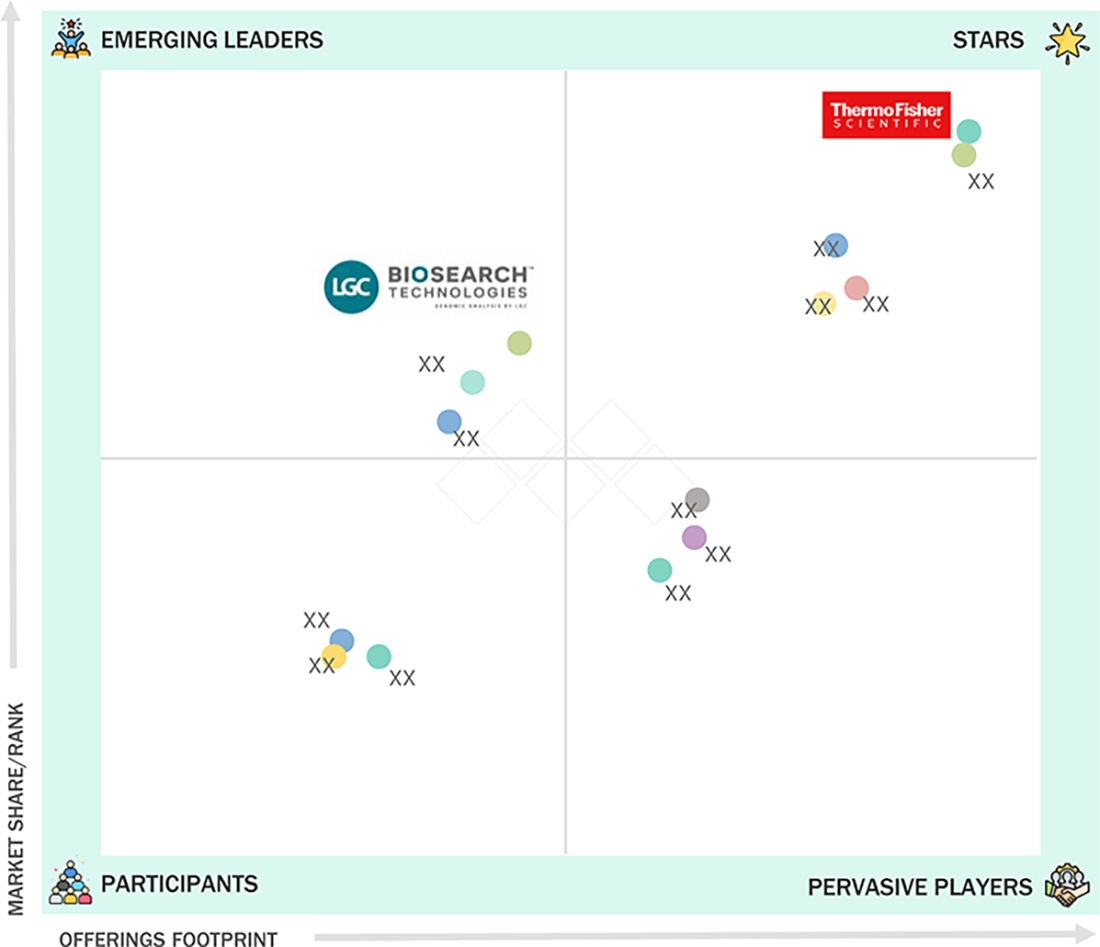
NORTH AMERICA NUCLEIC ACID ISOLATION AND PURIFICATION MARKET: COMPANY EVALUATION MATRIX
In the North America nucleic acid isolation and purification market matrix, Thermo Fisher Scientific (star) is a key player with deep penetration across clinical, research, and biopharma labs. Its portfolio spans magnetic bead- and column-based extraction kits, automated sample prep systems, and supporting buffers and plastics, making it a preferred choice for high-throughput PCR and NGS workflows. LGC Biosearch Technologies (emerging leader) is building momentum by enabling high-performance nucleic acid workflows that are directly part of the downstream process of extraction, especially for genomics and applied molecular testing.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Thermo Fisher Scientific, Inc. (US)
- Danaher Corporation (US)
- QIAGEN (Germany)
- F. Hoffman-La Roche (Switzerland)
- Agilent Technologies, Inc. (US)
- Illumina, Inc. (US)
- Merck KGaA (Germany)
- Bio-Rad Laboratories Inc. (US)
- Promega Corporation (US)
- New England Biolabs (US)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.80 Billion |
| Market Forecast in 2030 (Value) | USD 4.64 Billion |
| Growth Rate | CAGR of 9.3% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Million/Billion) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | US, Canada |
| Parent & Related Segment Reports |
Nucleic Acid Isolation & Purification Market Asia Pacific Nucleic Acid Isolation and Purification Market Europe Nucleic Acid Isolation and Purification Market Nucleic Acid Isolation and Purification Kits Market |
WHAT IS IN IT FOR YOU: NORTH AMERICA NUCLEIC ACID ISOLATION AND PURIFICATION MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| US-based diagnostics company |
|
|
RECENT DEVELOPMENTS
- July 2023 : INOVIQ and Promega Corporation announced a global joint marketing agreement for EXO-NET exosome isolation and nucleic acid purification solutions.
- October 2022 : Thermo Fisher Scientific Inc. (US) invested USD 97 million in expanding its clinical research operations in Richmond, Virginia. The facility will provide high-quality bioanalytical lab services to advance clinical research programs.
- May 2022 : Thermo Fisher Scientific Inc. (US) partnered with LabShares to provide instruments, lab equipment, and consumables to help early-stage life science companies accelerate their drug discovery efforts.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the North America nucleic acid isolation and purification market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The secondary sources referred for this research study include publications from government sources such as the World Health Organization (WHO), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), European Society of Human Genetics (ESHG), University Hospitals Southampton NHS Foundation Trust, Genomics England, Scottish Genome Partnership, Berlin Institute of Health, Max Delbrück Center for Molecular Medicine, and European Federation of Biotechnology (EFB). Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentations, Journals, Expert Interviews, and MarketsandMarkets Analysis. Secondary data was collected and analyzed to arrive at the overall size of the market, which was then validated by primary research.
Primary Research
Primary research was conducted to validate the market segmentation, identify key players, and gather insights into key industry trends and market dynamics.
Extensive primary research was conducted after acquiring basic knowledge about the market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side (hospitals and diagnostics centers, academic institutes and government organizations, pharmaceutical and biotechnology companies, and others) and supply side (C-level and D-level executives, product managers, and marketing and sales managers of key manufacturers, distributors, and channel partners, among others, from Tier 1 and Tier 2 companies engaged in offering products). The primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The report presents a detailed assessment of the North America nucleic acid isolation and purification market and qualitative inputs and insights from MarketsandMarkets. The total market size was determined after data triangulation from three different approaches. After completing each approach, the weighted average of the three approaches was taken based on each approach’s level of assumptions. A detailed market estimation approach was followed to estimate and validate the value of the market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- From the list of manufacturers in the market, companies were identified and finalized from secondary research.
- Revenues for individual companies were gathered from public sources and databases.
- The business shares of leading players were gathered from secondary sources to the extent available. In certain cases, the shares of product businesses were ascertained through a detailed analysis of various parameters, including product portfolios and market positioning.
- Individual shares or revenue estimates were validated through expert interviews.
Approach: MnM Repository Analysis
For the estimation of the North America nucleic acid isolation and purification market, related market reports in the MnM repository were considered. the regional/country-level market values of North America nucleic acid isolation and purification and dependent submarkets were extracted from the MnM repository and validated through secondary and primary research. The final market size was triangulated through the average of all approaches and validated through primary interviews with industry experts.
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. Data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments.
Market Definition:
Nucleic acid isolation and purification is a set of molecular biology techniques used for the extraction of DNA and RNA for use in downstream applications, such as sequencing, cloning, and polymerase chain reaction. These downstream applications are important parts of life science research, molecular diagnostics, forensics, and genetic engineering.
Key Stakeholders
- Manufacturers and vendors of North America nucleic acid isolation and purification kits and instruments
- Research associations related to genomics
- Hospitals & diagnostic centers
- Contract research organizations
- Healthcare institutions
- Research institutes
- Pharmaceutical & biotechnology companies
- Government & academic institutes
- Community centers
Report Objectives
- To define, describe, and forecast the North America nucleic acid isolation and purification market by product, method, type, application, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micro markets with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments, such as acquisitions, product launches, expansions, agreements, partnerships, and collaborations, in the market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Geographical Analysis
- Further breakdown of the RoE market, by country
- Further breakdown of the RoAPAC market, by country
- Further breakdown of the RoLATAM market, by country
Company Information
- Detailed analysis and profiling of additional market players (Up to five)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the North America Nucleic Acid Isolation and Purification Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation












Growth opportunities and latent adjacency in North America Nucleic Acid Isolation and Purification Market