
Nucleic Acid Isolation and Purification Kits Market Size, Growth, Share & Trends Analysis
Nucleic Acid Isolation and Purification Kits Market by Type (DNA, RNA, Total Nucleic Acid, Others), Method (Column, Magnetic Beads, Reagent), Application (Diagnostics, Personalized Medicine), End User (Pharma & Biotech, CROs) – Global Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
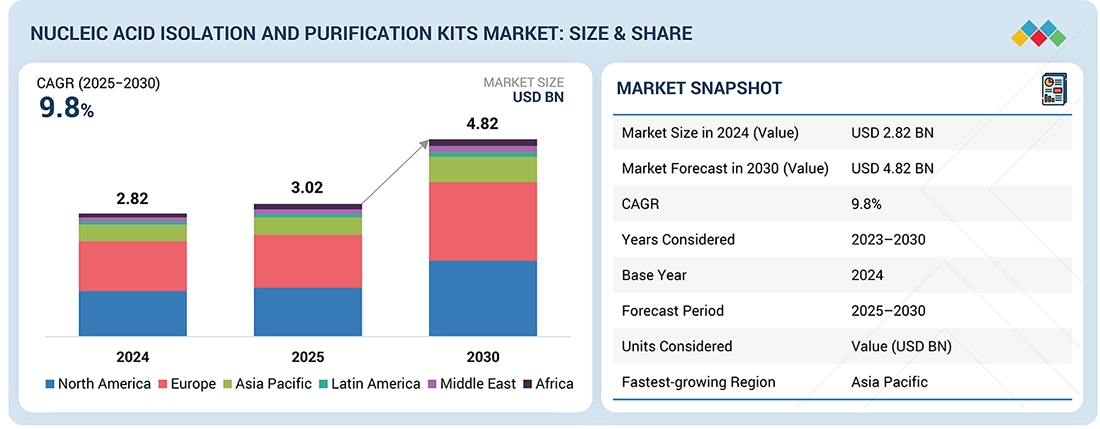
The global nucleic acid isolation and purification kits market, valued at US$2.82 billion in 2024, stood at US$3.02 billion in 2025 and is projected to advance at a resilient CAGR of 9.8% from 2025 to 2030, culminating in a forecasted valuation of US$4.82 billion by the end of the period. The growth of the nucleic acid isolation and purification kits market is mainly driven by the growth in molecular diagnostics and sequencing-based testing. Labs are running more PCR/RT-PCR panels, syndromic testing, and oncology and genetic assays. This directly aids in the growth of consumption of extraction kits across routine workflows.
KEY TAKEAWAYS
-
By RegionNorth America accounted for a 43.0% revenue share of the nucleic acid isolation and purification kits market in 2024.
-
By TypeBy type, the DNA extraction kits segment accounted for the largest share of the nucleic acid isolation and purification kits market in 2024.
-
By MethodBy method, the magnetic bead-based method segment dominated the market in 2024.
-
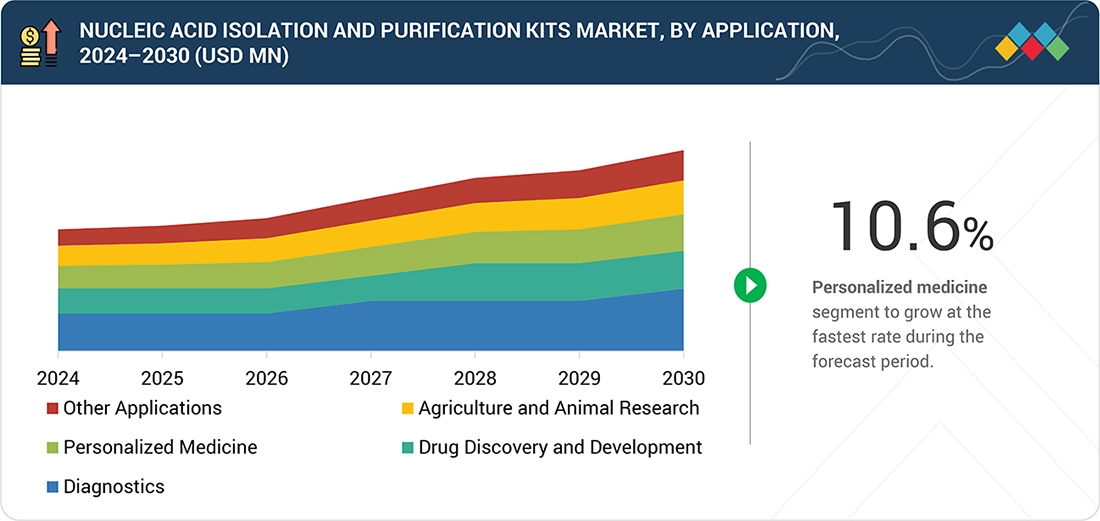
By ApplicationBy application, the personalized medicine segment is projected to grow at the fastest rate of 10.5% from 2025 to 2030.
-
By End userBy end user, the pharmaceutical & biotechnology companies segment is projected to grow at the fastest rate of 11.0% from 2025 to 2030.
-
Competitive LandscapeCompanies such as Thermo Fisher Scientific, QIAGEN, and F. Hoffmann-La Roche were identified as key players in the nucleic acid isolation and purification kits market, given their strong market share and extensive product footprint.
-
Competitive LandscapeCompanies such as HiMedia Laboratories and Caisson Labs, among others, have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders.
The nucleic acid isolation and purification kits market is expanding steadily as more laboratories require reliable and repeatable extraction for routine and high-volume workflows. Demand is increasing from hospital labs, reference labs, public health labs, and research centers that routinely run large numbers of molecular tests. There is also an observed shift toward standardized, automation-ready extraction. This is aiding innovation and growth in the market. Additionally, with a widening sample mix, the adoption and demand for specialty kits are growing.
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The impact on stakeholders in the market for nucleic acid isolation and purification kits is largely influenced by the quick expansion of molecular diagnostics, NGS-based research, and decentralized testing models. Labs performing infectious disease panels, oncology profiling, and genetic testing require extraction kits that provide a consistent yield and purity for high sample volumes. Additionally, a large number of labs are normalizing their workflows to reduce variability and shorten turnaround times. Consequently, the increased use of automation, compatible kits, magnetic bead chemistries, and ready-to-use formats, which are suitable for both routine clinical operations and research pipelines, is being facilitated.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Clinical NGS expansion in oncology and precision testing

-
Labs demand for standardization and automation-ready workflows
Level
-
Cost pressure on labs and per-sample reagent spend
Level
-
Automation-first magnetic bead kits and high-throughput formats
Level
-
Wide sample diversity and inhibitors that hit yield and purity
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Clinical NGS expansion in oncology and precision testing
Tumor profiling is shifting to routine use in many centers. Guidelines increasingly recommend NGS in several advanced cancers. As NGS testing expands, upstream sample prep is becoming a core requirement. Labs need consistent yield and purity to avoid sequencing failure. This supports steady demand for extraction and clean-up kits. It is also increasing the use of higher-performance kits for low-input and mixed samples.
Restraint: Cost pressure on labs and per-sample reagent spend
Extraction is a recurring cost in molecular workflows. Labs often face fixed budgets and strict cost-per-test targets. When volumes spike, the spend increases significantly. For instance, during COVID-scale demand, many labs reported limits from kit availability and cost. This can delay upgrades or push labs to adopt lower-cost alternatives. It also shifts buying toward tenders and aggressive price competition.
Opportunity: Automation-first magnetic bead kits and high-throughput formats
Laboratories are increasingly transitioning to plate-based and automated extraction systems. Bead-based kits are highly compatible with robotics and standardized protocols, paving the way for fully automated workflows and ready-to-use reagents. Vendors can gain a competitive advantage by enhancing speed, yield, and contamination control. Additionally, there is a growing demand for instrument-agnostic kits that are compatible with various platforms. Consequently, forming automation partnerships and validating workflows can help expand market share.
Challenge: Wide sample diversity and inhibitors that hit yield and purity
Laboratories analyze various samples, including swabs, blood, plasma, stool, saliva, and tissue. Each sample type has its own set of inhibitors and background contaminants, which can complicate results. Low-input and degraded samples increase the risk of failure. Formalin-Fixed Paraffin-Embedded (FFPE) samples often present challenges due to their lower-quality nucleic acids. Testing kits must effectively balance yield, purity, and speed across these different sample types, making it difficult to achieve consistent performance.
NUCLEIC ACID ISOLATION AND PURIFICATION KITS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
NHM’s Core Labs team processes highly diverse and often contaminated samples for sequencing-based identification. The lab frequently uses QIAGEN nucleic acid extraction kits and highlights the DNeasy Blood & Tissue Kit for getting high-quality DNA from a variety of samples. | Reliable DNA yield across many tissue types | Better success on degraded/low-input samples | Fewer repeat extractions and smoother sequencing throughput |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The nucleic acid isolation and purification kits market is a connected ecosystem that includes chemical and consumable suppliers, kit manufacturers, automation partners, distributors, and end users across clinical and research settings. Upstream, the value chain starts with suppliers of magnetic beads/silica matrices, resins, membranes, and buffers, among others. It also included suppliers of plastics such as columns, tubes, tips, and plates. It also includes partners supplying sterile packaging, labels, and cold-chain logistics for sensitive components. On the demand side, key users include hospitals and diagnostic laboratories, reference labs, public health labs, blood banks, CROs, and biopharma and biotech research end users.
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Nucleic Acid Isolation and Purification Kits Market, by Type
In 2024, the DNA extraction kits segment dominated the nucleic acid isolation and purification kits market. This is mainly because a wide range of high-volume workflows rely on DNA preparation as the first step. Routine clinical molecular testing, genetic screening, and oncology assays often begin with the extraction of genomic DNA from blood, saliva, and tissue. DNA is also more stable than RNA, making handling and storage easier in routine laboratory operations.
Nucleic Acid Isolation and Purification Kits Market, by Application
In 2024, diagnostics is the dominant application in the nucleic acid isolation and purification kits market. This is mainly because clinical labs run extraction as a routine step before PCR/RT-PCR and other molecular assays. Infectious disease testing remains a high-volume driver, supported by respiratory panels, STI testing, and hospital-acquired infection screening.
Nucleic Acid Isolation and Purification Kits Market, by End User
In 2024, hospitals and diagnostic centers were at the forefront of the nucleic acid isolation and purification kits market. This is primarily due to the fact that they are the facilities that perform the highest routine volume of patient samples for PCR/RT, PCR, and other molecular diagnostic tests. Extraction workflows are kept running continuously, for example, through routine infectious disease testing and pre-surgical screening.
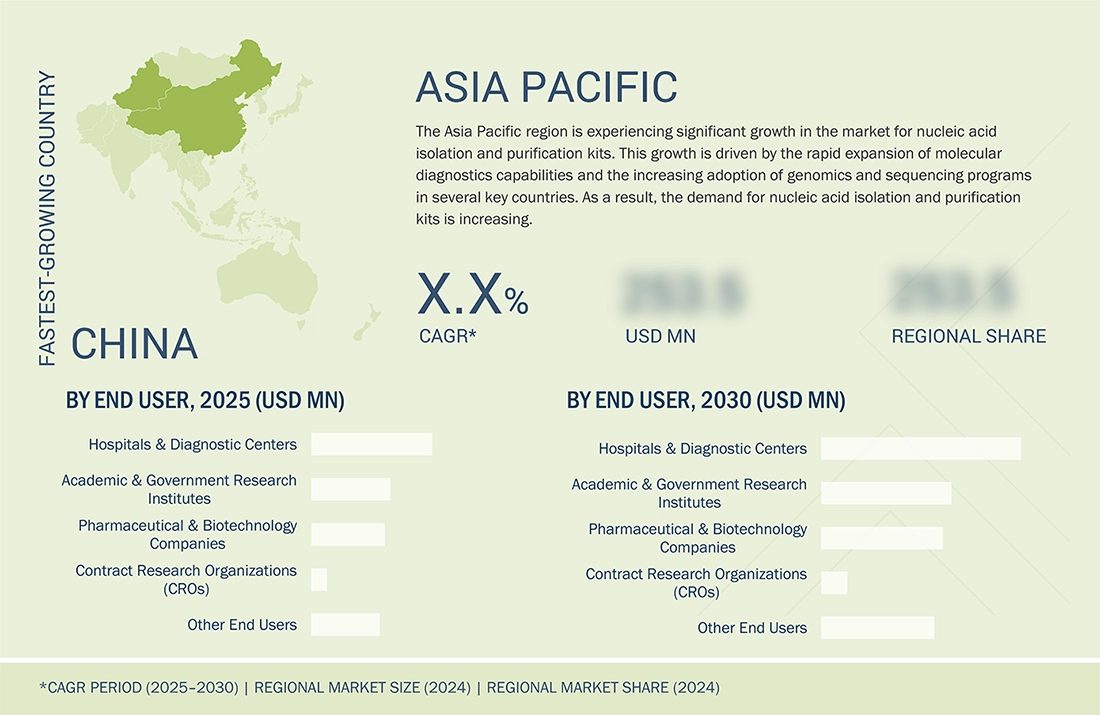
REGION
Asia Pacific to be the fastest-growing region in the nucleic acid isolation and purification kits market during the forecast period
The nucleic acid isolation and purification kits market in the Asia Pacific region is experiencing significant expansion. The increasing use of molecular diagnostics and the growing number of PCR and syndromic testing in countries such as China, Japan, India, South Korea, and Singapore are major contributors to the market growth in this region. The local spending on genomics, public health surveillance, and hospital lab capacity is leading to an increase in routine extraction volumes.
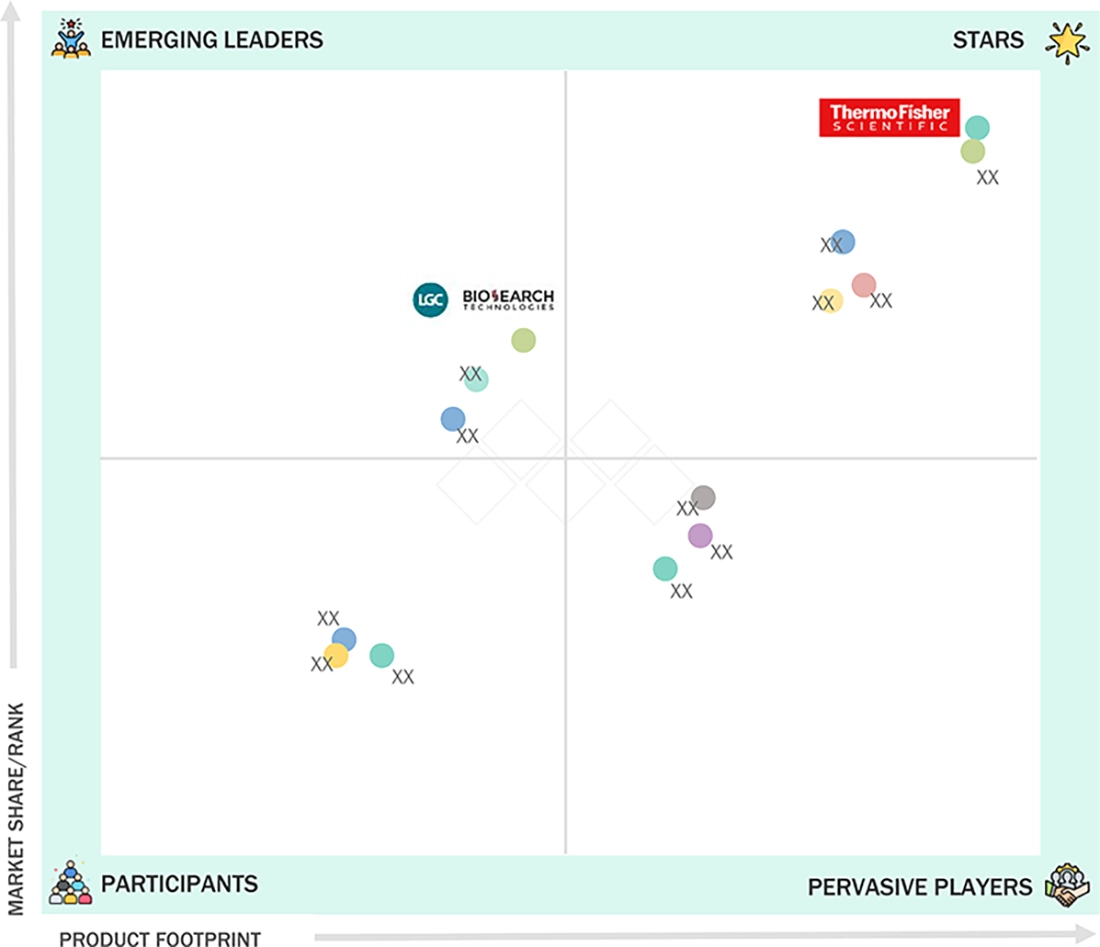
NUCLEIC ACID ISOLATION AND PURIFICATION KITS MARKET: COMPANY EVALUATION MATRIX
Thermo Fisher Scientific (Star) leads the nucleic acid isolation and purification kits market through a broad, lab-ready portfolio used in both diagnostics and research. Its MagMAX extraction kits are widely used for high-throughput DNA/RNA workflows. The company also benefits from strong pull-through via KingFisher automated extraction systems. This creates repeat kit demand across hospital labs, reference labs, and biopharma testing teams. LGC Biosearch Technologies (Emerging Leader) is strengthening its position through magnetic bead chemistries that fit modern nucleic acid workflow standardization.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- Thermo Fisher Scientific, Inc. (US)
- QIAGEN (Germany)
- Merck KGaA (Germany)
- F. Hoffman-La Roche Ltd. (Switzerland)
- Bio-Rad Laboratories (US)
- Promega Corporation (US)
- Danaher Corporation (US)
- Agilent Technologies (US)
- LGC Biosearch Technologies (UK)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 2.82 Billion |
| Market Forecast in 2030 (Value) | USD 4.82 Billion |
| Growth Rate | CAGR of 9.8% from 2025–2030 |
| Years Considered | 2023–2030 |
| Base Year | 2024 |
| Forecast Period | 2025–2030 |
| Units Considered | Value (USD Billion) |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
|
| Regions Covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
| Parent & Related Segment Reports |
Nucleic Acid Isolation & Purification Market North America Nucleic Acid Isolation and Purification Market Asia Pacific Nucleic Acid Isolation and Purification Market Europe Nucleic Acid Isolation and Purification Market |
WHAT IS IN IT FOR YOU: NUCLEIC ACID ISOLATION AND PURIFICATION KITS MARKET REPORT CONTENT GUIDE
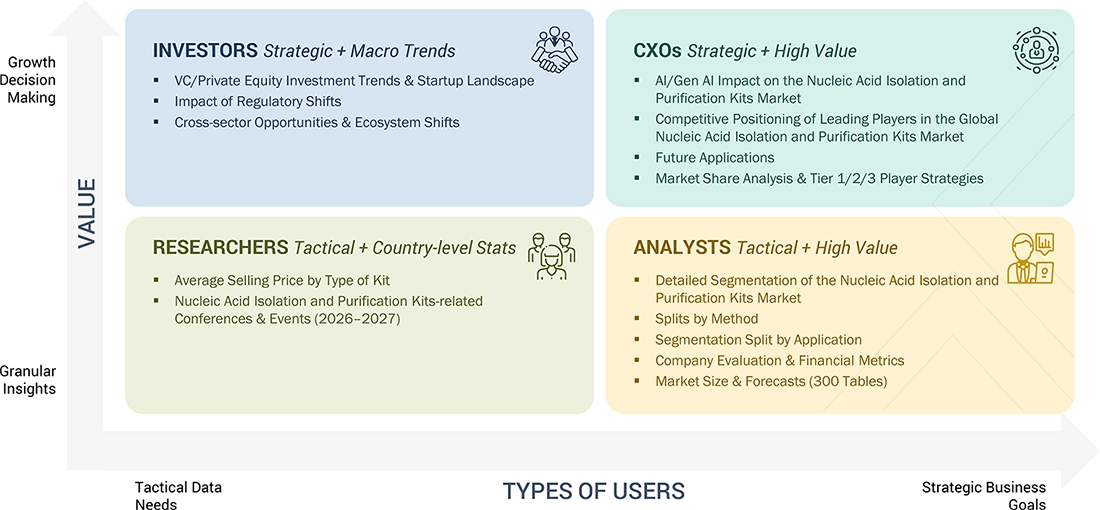
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Hospital lab/diagnostic network scaling molecular testing |
|
|
RECENT DEVELOPMENTS
- September 2023 : QIAGEN launched two new nucleic acid extraction kits: the QIAwave RNeasy Plus Mini Kit and QIAwave DNA/RNA Mini Kit, eco-friendlier versions of the RNeasy Plus Mini Kit and the All DNA/RNA Mini Kit.
- July 2023 : INOVIQ and Promega Corporation announced a global joint marketing agreement for EXO-NET exosome isolation and nucleic acid purification solutions.
Table of Contents

Methodology
This research study involved the extensive use of secondary sources, directories, and databases to identify and collect valuable information for the analysis of the nucleic acid isolation and purification kit market. In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The global market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
The secondary sources referred for this research study include publications from government sources such as the World Health Organization (WHO), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), European Society of Human Genetics (ESHG), University Hospitals Southampton NHS Foundation Trust, Genomics England, Scottish Genome Partnership, Berlin Institute of Health, Max Delbrück Center for Molecular Medicine, and European Federation of Biotechnology (EFB). Company Websites, Annual Reports, SEC Filings, Press Releases, Investor Presentations, Journals, Expert Interviews, and MarketsandMarkets Analysis. Secondary data was collected and analyzed to arrive at the overall size of the global market, which was then validated by primary research.
Primary Research
Primary research was conducted to validate the market segmentation, identify key players, and gather insights into key industry trends and market dynamics.
Extensive primary research was conducted after acquiring basic knowledge about the global market scenario through secondary research. Several primary interviews were conducted with market experts from the demand side (hospitals and diagnostics centers, academic institutes and government organizations, pharmaceutical and biotechnology companies, and others) and supply side (C-level and D-level executives, product managers, and marketing and sales managers of key manufacturers, distributors, and channel partners, among others, from Tier 1 and Tier 2 companies engaged in offering products) across five major regions, namely, North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. Approximately 80% of primary interviews were conducted with supply-side representatives, while demand-side participants accounted for the remaining share. The primary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
To know about the assumptions considered for the study, download the pdf brochure
Market Size Estimation
The report presents a detailed assessment of the nucleic acid isolation and purification kit market and qualitative inputs and insights from MarketsandMarkets. The total market size was determined after data triangulation from three different approaches. After completing each approach, the weighted average of the three approaches was taken based on each approach’s level of assumptions. A detailed market estimation approach was followed to estimate and validate the value of the market and other dependent submarkets. These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
- From the list of manufacturers in the market, companies were identified and finalized from secondary research.
- Revenues for individual companies were gathered from public sources and databases.
- The business shares of leading players were gathered from secondary sources to the extent available. In certain cases, the shares of product businesses were ascertained through a detailed analysis of various parameters, including product portfolios and market positioning.
- Individual shares or revenue estimates were validated through expert interviews.
Approach: MnM Repository Analysis
For the estimation of the nucleic acid isolation and purification kit market, related market reports in the MnM repository were considered. The global and regional market values of the nucleic acid isolation and purification kit and dependent submarkets were extracted from the MnM repository and validated through secondary and primary research. The final global market size was triangulated through the average of all approaches and validated through primary interviews with industry experts.
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. Data triangulation and market breakdown procedures were employed, wherever applicable, to complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments.
Market Definition:
nucleic acid isolation and purification kit is a set of molecular biology techniques used for the extraction of DNA and RNA for use in downstream applications, such as sequencing, cloning, and polymerase chain reaction. These downstream applications are important parts of life science research, molecular diagnostics, forensics, and genetic engineering.
Key Stakeholders
- Manufacturers and vendors of nucleic acid isolation and purification kit kits and instruments
- Research associations related to genomics
- Hospitals & diagnostic centers
- Contract research organizations
- Healthcare institutions
- Research institutes
- Pharmaceutical & biotechnology companies
- Government & academic institutes
- Community centers
Report Objectives
- To define, describe, and forecast the nucleic acid isolation and purification kit market by product, method, type, application, end user, and region
- To provide detailed information regarding the major factors influencing the market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micro markets with respect to individual growth trends, future prospects, and contributions to the overall market
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To forecast the size of the market segments with respect to five main regions: North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa
- To profile the key players and comprehensively analyze their product portfolios, market positions, and core competencies
- To track and analyze competitive developments, such as acquisitions, product launches, expansions, agreements, partnerships, and collaborations, in the global market
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for this report:
Geographical Analysis
- Further breakdown of the RoAPAC market, by country
Company Information
- Detailed analysis and profiling of additional market players (Up to five)
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Nucleic Acid Isolation and Purification Kits Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Nucleic Acid Isolation and Purification Kits Market