
Europe Biomarkers Market Size, Growth, Share & Trends Analysis
Europe Biomarkers Market by Offering (Consumables, Software, Services), Type (Safety, Efficacy), Research Area (Genomics, Proteomics), Technology (PCR, MS), Disease (Cancer, Infectious), Application (Diagnostic, Research)- Forecast to 2030




OVERVIEW
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
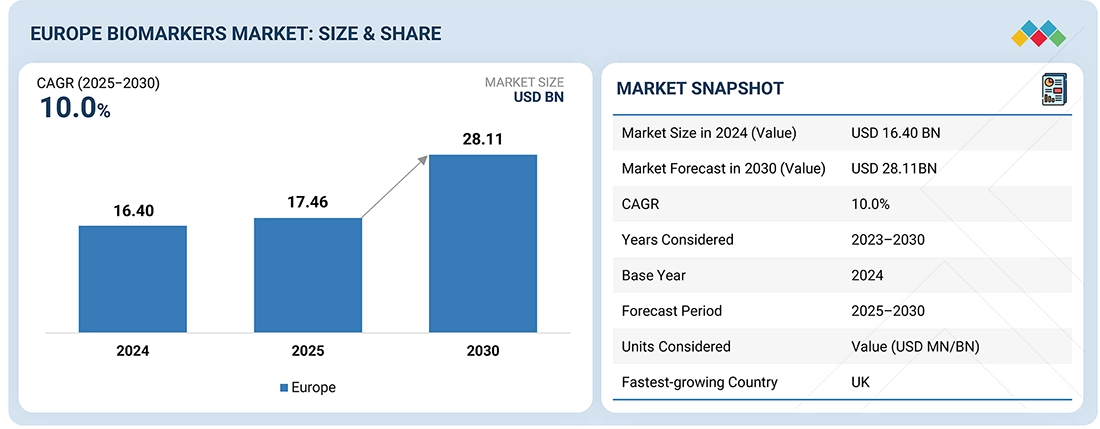
The Europe biomarkers Market, valued at US$16.40 billion in 2024, stood at US$17.46 billion in 2025 and is projected to advance at a resilient CAGR of 10.0% from 2025 to 2030, culminating in a forecasted valuation of US$28.11 billion by the end of the period. A biomarker (short for biological marker) is a measurable indicator of a biological condition or process, often used to diagnose diseases, predict disease progression, and evaluate treatment response; this market includes consumables, services, and software used for biomarker-related research.
KEY TAKEAWAYS
-
BY REGIONThe UK biomarkers market was the fastest growing segment with CAGR 11.7% in 2024.
-
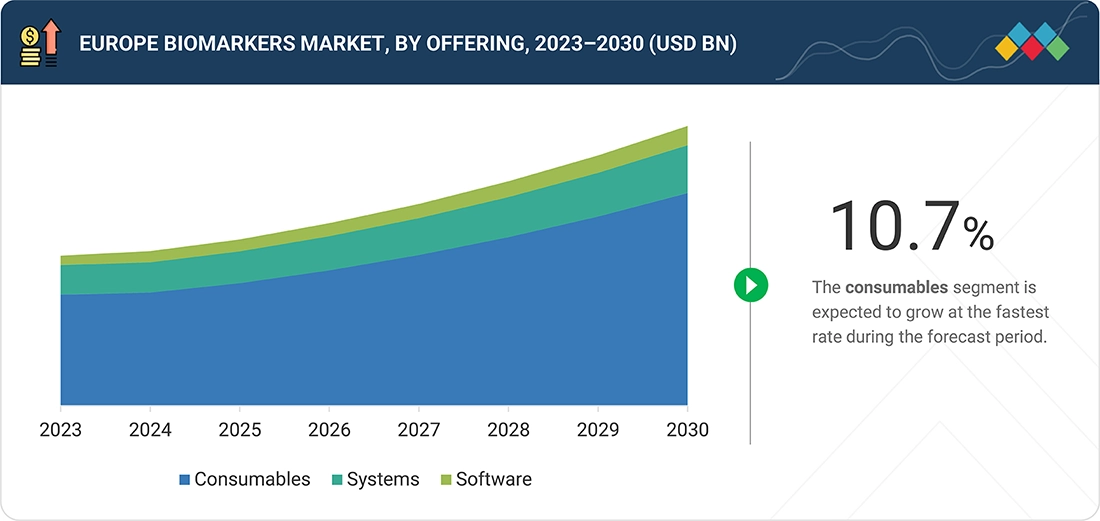
BY OfferingsBy offering, the consumables segment is the highest growing segment with CAGR 10.7 % in 2024
-
BY TYPEBy type, the safety biomarker segment dominate the Europe biomarkers market with a CAGR of 10.6%.
-
BY Research areaBy research area, the genomics segment is expected to dominate the market during the study period.
-
COMPETITIVE LANDSCAPERoche (Switzerland), Merck KGAA (Germany), and QIAGEN (Germany) were identified as some of the star players in the Europe biomarkers market, given their strong market share and product footprint.
-
COMPETITIVE LANDSCAPEBiocartis NV (Belgium),Centogene GMBH (Germany), and Scailtye AG (Switzerland) have distinguished themselves among startups and SMEs by securing strong footholds in specialized niche areas, underscoring their potential as emerging market leaders
The Europe biomarkers market is expanding rapidly, driven by the surge in precision-medicine programs and the routine use of biomarker-guided tests in oncology, cardiology, and other complex diseases.
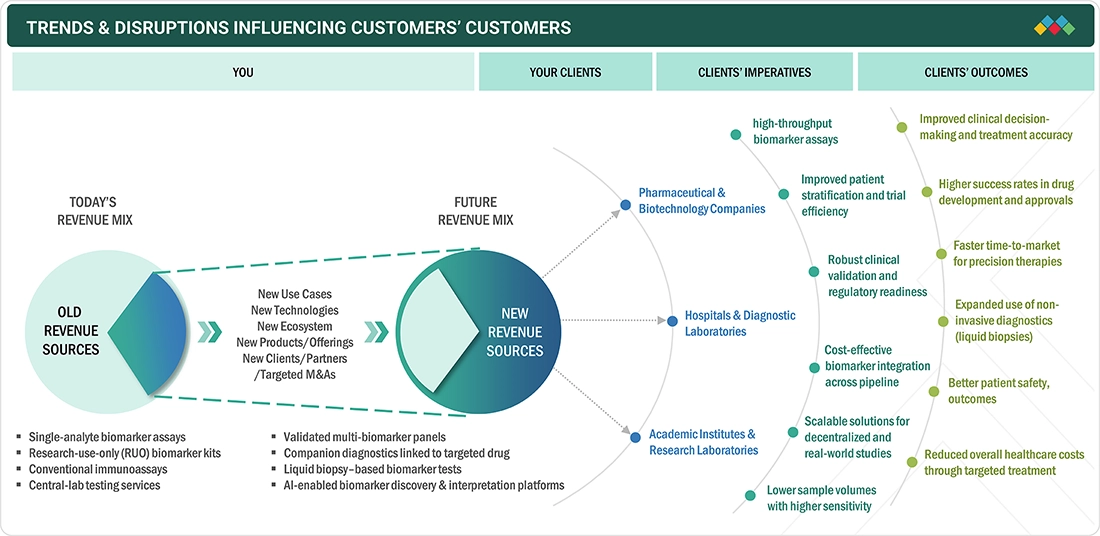
TRENDS & DISRUPTIONS IMPACTING CUSTOMERS' CUSTOMERS
The biomarkers market in Europe is undergoing rapid transformation, with trends in manufacturing, regulation, and therapeutic applications shifting to support precision medicine and complex biologic therapies. High volumes of cancer, cardiovascular, and neurological testing are fueling demand for high-performance assay kit solutions, multiplex testing solutions, and single-sample multi-omics solutions that deliver consistent, clinically relevant results. Hospitals, lab corporations, and CDMO organizations are adopting validated, automation-ready biomarker consumables and solutions that provide rapid turnaround times, support companion diagnostics, and meet the evolving expectations of the EMA and end users.
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
MARKET DYNAMICS
Level
-
Rising burden of chronic and age-related diseases

-
Strong focus on precision and personalized medicine across Europe
Level
-
Complex and evolving regulatory landscape
Level
-
Expansion of companion diagnostics alongside targeted therapie & precision therapies
Level
-
Data privacy and governance issues under GDPR
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Driver: Rising burden of chronic and age-related diseases
The rising burden of chronic and age-related diseases is pushing clinicians to switch toward genomic biomarkers to detect cancer and other conditions earlier, to choose targeted therapies, and to track how well patients respond over time.
Restraint: Complex and evolving regulatory landscape
A complex and constantly evolving regulatory landscape for genomic tests and companion diagnostics makes it more challenging and time-consuming for labs and companies to bring new biomarker assays into routine clinical use.
Opportunity: Expansion of companion diagnostics alongside targeted therapie & precision therapies
The growth of companion diagnostics alongside targeted and precision therapies is creating strong demand for validated genomic biomarker panels that directly link test results to specific drugs and clinical trial options.
challenges : Data privacy and governance issues under GDPR
Data privacy and governance requirements, including GDPR and similar rules, add pressure on labs and test developers to manage genomic data securely while still enabling sharing for research, clinical decision-support, and real-world evidence generation.
EUROPE BIOMARKERS MARKET: COMMERCIAL USE CASES ACROSS INDUSTRIES
| COMPANY | USE CASE DESCRIPTION | BENEFITS |
|---|---|---|
 |
Foundation Medicine tissue and liquid comprehensive genomic profiling tests used by oncology centers to identify actionable mutations, complex signatures (TMB, MSI), and minimal residual disease across solid tumors. | Delivers a broad view of a patient’s tumor biology from limited sample, helping clinicians select targeted and immunotherapies, enroll patients in trials, and monitor relapse risk with a single CGP-driven workflow |
 |
QIAseq and GeneRead oncology panels, plus qPCR/dPCR assays and genomic services, used by pharma, hematology labs, and academic centers for blood cancer biomarkers, immuno-oncology markers, and companion-diagnostic development. | Provides flexible sample-to-insight workflows that combine sensitive mutation detection with curated interpretation, shortening biomarker discovery timelines and making it easier to translate research panels into regulated clinical assays |
Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET ECOSYSTEM
The Europe biomarker market represents a highly integrated community of test developers, kit and instrument suppliers, clinical labs, hospitals, and biopharma. Manufacturers provide the necessary biomarker assays and platforms, while pharma, CROs, and CDMOs incorporate them into trial design, patient stratification, and therapeutic response measurement. Finally, hospitals and referral labs incorporate these into healthcare practice, with support from IT companies and distribution channels.

Logos and trademarks shown above are the property of their respective owners. Their use here is for informational and illustrative purposes only.
MARKET SEGMENTS
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
Europe Biomarkers Market, By offerings
In 2024, consumables accounted for the largest share of the Europe biomarkers market, reflecting the heavy, recurring use of assay kits, reagents, plates, and cartridges in both clinical testing and large research studies
Europe Biomarkers Market, By Type
In 2024, the safety biomarkers segment accounted for largest market share, reflecting their growing use to monitor organ toxicity and adverse drug reactions in real time during clinical trials and post-marketing surveillance.
Europe Biomarkers Market, By Research area
In?????? 2024, genomics held the major share by research area, with next-generation sequencing and gene panels widely used to uncover actionable variants and build composite biomarker signatures for cancer and rare disease programs
Europe Biomarkers Market, By Technology
In 2024, immunoassay technologies accounted for the largest share due to automation-friendliness, and suitability for high-volume routine testing in hospitals and reference laboratories
Europe Biomarkers Market, By Disease indication
In 2024, cancer commanded the largest market share due to the demand of molecular and protein markers for early detection, minimal residual disease assessment, and therapy monitoring.
Europe Biomarkers Market, By Application
Clinical diagnostics had the highest share of the Europe biomarkers market in 2024, due to the increased demand for biomarker-based assays by hospitals and reference laboratories for cancer, cardiovascular disease, and infections.
Europe Biomarkers Market, By End user
Pharmaceutical & biotechnology companies were the highest end user segment, as they use biomarkers to de-risk pipelines, optimise trial design, and get approval for diagnostics that are launched along with new therapies.
REGION
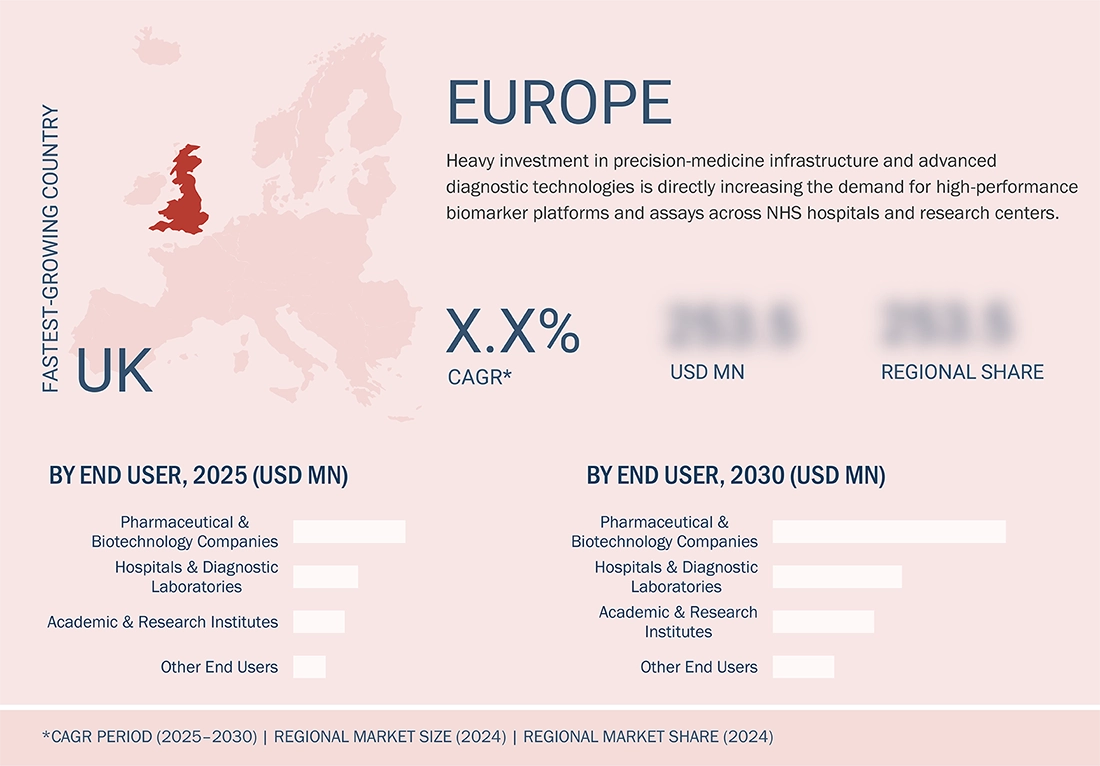
US to be fastest-growing country in market during forecast period
In 2024, the UK was the fastest-growing country in Europe biomarkers market, supported by a network of academic and research centers, cancer institutes, and innovation-driven hospitals. A growing pipeline of oncology and autoimmune drugs, along with increased investment in precision medicine initiatives and large-scale clinical trials, is driving demand for biomarker assays and companion diagnostics across genomics, proteomics, and immunoassay platforms.rms.
EUROPE BIOMARKERS MARKET: COMPANY EVALUATION MATRIX
In the Europe biomarkers market, Roche (Star Player) offers a range of assays, immunoassay analyzers, and point-of-care systems that laboratories trust for high-volume routine testing and for cardiac, infectious, and metabolic panels. Biomerieux (Emerging Leader) is on the genomics side, combining NGS instruments, oncology panels, and powerful analytics to turn complex genomic biomarkers into practical tools for cancer profiling and personalized treatment decisions across leading European centers.
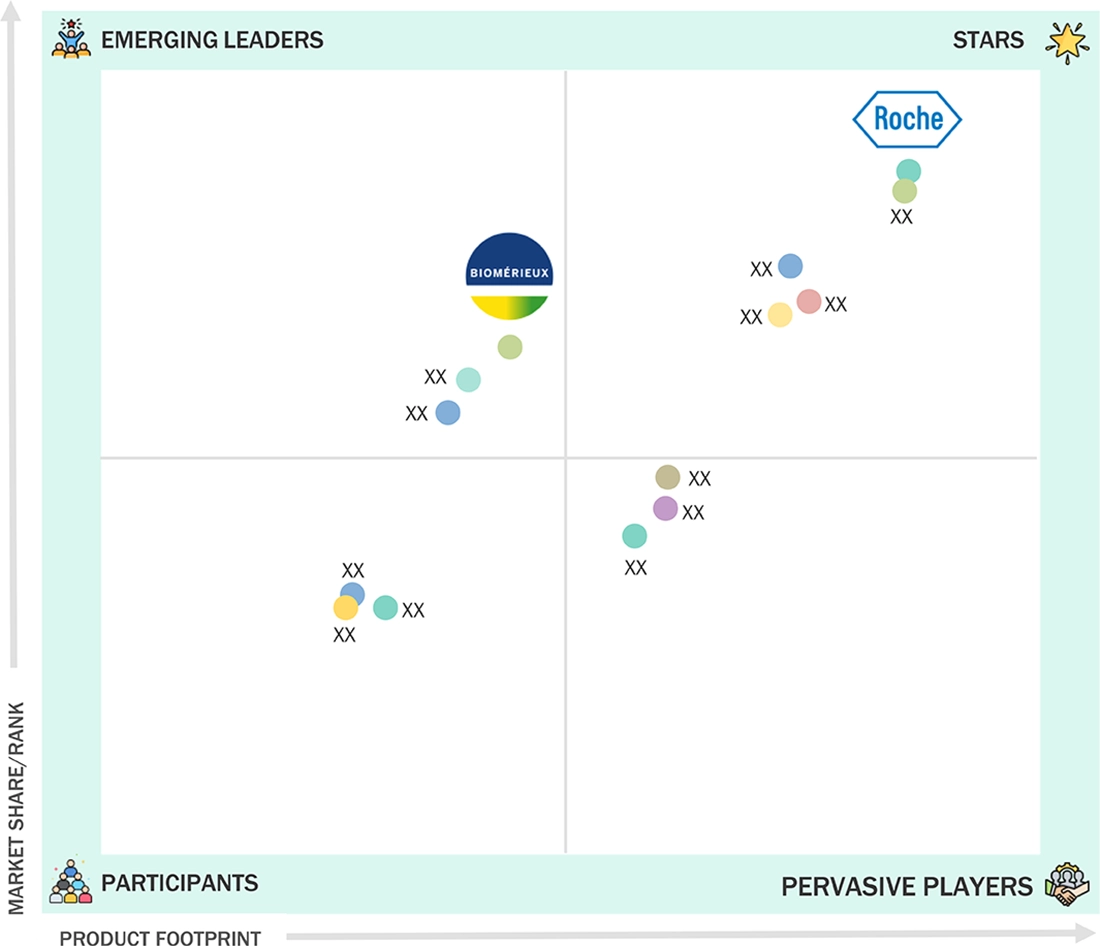
Source: Secondary Research, Interviews with Experts, MarketsandMarkets Analysis
KEY MARKET PLAYERS
- F. Hoffmann-La Roche Ltd (Switzerland)
- Merck KGaA (Germany)
- QIAGEN (Germany)
- Eurofins Scientific (Luxembourg)
- bioMérieux (France)
- R-Biopharm AG (Germany)
- Synexa Life Sciences BV (Netherlands)
- Proteome Sciences (UK)
MARKET SCOPE
| REPORT METRIC | DETAILS |
|---|---|
| Market Size in 2024 (Value) | USD 16.40 Billion |
| Market Forecast in 2030 (Value) | USD 28.10 Billion |
| Growth Rate | CAGR of 10.0% from 2025-2030 |
| Years Considered | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Million) |
| Report Coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments Covered |
|
| Countries Covered | Germany, UK, France, Italy, Spain, Rest of Europe |
| Parent & Related Segment Reports |
Biomarkers Market North America Biomarkers Market Asia Pacific Biomarkers Market Genomic Biomarkers Market Predictive Clinical Biomarkers Market Neurological Biomarkers Market Digital Biomarker Market EPO Biomarkers Market |
WHAT IS IN IT FOR YOU: EUROPE BIOMARKERS MARKET REPORT CONTENT GUIDE
DELIVERED CUSTOMIZATIONS
We have successfully delivered the following deep-dive customizations:
| CLIENT REQUEST | CUSTOMIZATION DELIVERED | VALUE ADDS |
|---|---|---|
| Sustainability-focused biomarker testing roadmap | Assessed the shift across European labs toward low-volume, high-information biomarker assays and digital readouts that reduce reagent consumption, plastic use, and sample waste. | Supports ESG narratives for European customers and helps position biomarker portfolios as greener, more efficient testing options for national health systems and private labs. |
| Benchmarking end-user technology preferences | Compared adoption of immunoassays, NGS, multiplex platforms, and point-of-care solutions across hospitals, reference labs, pharma, and academic centers in key European markets. | Guides platform roadmaps and sales focus, ensuring that the most relevant technologies are prioritized and promoted to each European customer segment. |
RECENT DEVELOPMENTS
- February 2024 : Roche committed to developing Al- enabled digital pathology algorithms for companion diagnostics.
- January 2025 : Merck entered a multi-year partnership with Opentrons Labworks to automate its biology assay kits on the Opentrons Flex robotic workstation. The collaboration is aimed at streamlining workflows for protein sample preparation and molecular assays, reducing hands-on time and improving lab productivity.
Table of Contents

Methodology
This research study extensively used secondary sources, directories, and databases to identify and collect valuable information to analyze the Europe Biomarkers Market . In-depth interviews were conducted with various primary respondents, including key industry participants, subject-matter experts (SMEs), C-level executives of key market players, and industry consultants, to obtain and verify critical qualitative and quantitative information and assess the growth prospects of the market. The market size estimated through secondary research was then triangulated with inputs from primary research to arrive at the final market size.
Secondary Research
Secondary research was used mainly to identify and collect information for the extensive, technical, market-oriented, and commercial study of the Europe Biomarkers Market . The secondary sources used for this study include American Society of Clinical Oncology (ASCO), Canadian Alliance for Healthy Hearts and Minds (CAHHM), Canadian Institute for Health Information (CIHI), Central Drugs Standard Control Organization (CDSCO), Center for Disease Evaluation and Research (CDER), Centers for Disease Control and Prevention (CDC), Chinese Medical Journal, Clinicaltrials.gov.in, European Medicines Agency (EMA), Food and Drug Administration (FDA), GLOBOCAN, International Agency for Research on Cancer (IARC), National Cancer Institute (NCI), National Center for Biotechnology Information (NCBI), National Institutes of Health (NIH), National Comprehensive Cancer Network (NCCN), Organization for Economic Co-operation and Development (OECD), Population Health Research Institute (PHRI), PubMed, World Bank, World Health Organization (WHO), Corporate and Regulatory Filings, Annual Reports, Sec Filings, Investor Presentations, and Financial Statements; Business Magazines & Research Journals; Press Releases, MarketsandMarkets Analysis. These sources were also used to obtain key information about major players, market classification, and segmentation according to industry trends, regional/country-level markets, market developments, and technology perspectives.
Primary Research
Extensive primary research was conducted after acquiring basic knowledge about the Europe Biomarkers Market scenario through secondary research. Approximately 70% of primary interviews were conducted with supply-side representatives, while demand-side participants accounted for the remaining share. This preliminary data was collected through questionnaires, e-mails, online surveys, personal interviews, and telephonic interviews.
Market Size Estimation
Both top-down and bottom-up approaches were used to estimate and validate the total size of the Europe Biomarkers Market . These methods were also used extensively to estimate the size of various subsegments in the market. The research methodology used to estimate the market size includes the following:
Bottom-up Approach
- The key players in the industry and market have been identified through extensive secondary research.
- The revenues generated from the Europe Biomarkers Market business of leading players have been determined through primary and secondary research.
- All percentage shares, splits, and breakdowns have been determined using secondary sources and verified through primary sources.
Top-down Approach
- After arriving at the overall market size from the market size estimation process, the total market was split into several segments and subsegments.
Data Triangulation
After arriving at the market size from the market size estimation process explained above, the total market was divided into several segments and subsegments. To complete the overall market engineering process and arrive at the exact statistics for all segments and subsegments, data triangulation and market breakdown procedures were employed, wherever applicable.
Market Definition
A biomarker is a measurable indicator of a biological condition or process, often used to diagnose diseases, predict disease progression, and evaluate treatment response. Biomarkers can be molecular, histologic, radiographic, or physiological characteristics that provide valuable insights into normal and pathological processes. The Europe Biomarkers Market encompasses a broad range of products and services that support biomarker discovery, validation, and application in clinical and research settings. This includes offerings such as consumables (assay kits, reagents, and instruments), software tools, and related services that facilitate biomarker analysis. The market report analyzes biomarker usage across multiple applications, including clinical diagnostics, drug discovery and development, personalized medicine, and clinical research. It also studies the adoption of biomarkers across key research areas, such as oncology, neurology, cardiology, infectious diseases, and metabolic disorders. Furthermore, the report segments the market by type of biomarkers, including safety, efficacy, and validation biomarkers. The study also considers regional trends, end-user adoption, and technological advancements shaping the biomarker landscape.
Stakeholders
- Academic & Research Institutes
- Biomarkers Assays and Reagents Manufacturers, Vendors, and Distributors
- Contract Research Organizations (CROs)
- Biomarkers Service & Software Providers
- Diagnostics Companies
- Market Research and Consulting Firms
- Pharmaceutical and Biotechnology Companies
- Regulatory Agencies
- Venture Capitalists
- Forensics Labs
- Government organizations
- Private research firms
- Contract development and manufacturing organizations (CDMOs)
- Hospitals and Diagnostic Laboratories
Report Objectives
- To define, describe, and forecast the Europe Biomarkers Market based on offering, type, research area, technology, disease indication, application, end user, and region
- To provide detailed information regarding the major factors influencing market growth (such as drivers, restraints, opportunities, and challenges)
- To strategically analyze micro-markets with respect to individual growth trends, prospects, and contributions to the overall market
- To analyze the opportunities in the market for stakeholders and provide details of the competitive landscape for market leaders
- To profile the key players in the Europe Biomarkers Market and comprehensively analyze their core competencies and market shares
- To track and analyze competitive developments such as acquisitions, product launches, expansions, agreements, partnerships, and collaborations in the Europe Biomarkers Market
- To benchmark players in the Europe Biomarkers Market using the “Company Evaluation Matrix” framework, which analyzes market players based on various parameters, including product portfolio, geographic reach, and market share
Need a Tailored Report?
Customize this report to your needs
Get 10% FREE Customization
Customize This ReportPersonalize This Research
- Triangulate with your Own Data
- Get Data as per your Format and Definition
- Gain a Deeper Dive on a Specific Application, Geography, Customer or Competitor
- Any level of Personalization
Let Us Help You
- What are the Known and Unknown Adjacencies Impacting the Europe Biomarkers Market
- What will your New Revenue Sources be?
- Who will be your Top Customer; what will make them switch?
- Defend your Market Share or Win Competitors
- Get a Scorecard for Target Partners
Custom Market Research Services
We Will Customise The Research For You, In Case The Report Listed Above Does Not Meet With Your Requirements
Get 10% Free Customisation











Growth opportunities and latent adjacency in Europe Biomarkers Market